Tutorial 12.10 - Integrated Nested Laplace Approximation (INLA) for (generalized) linear models
25 Feb 2016
Overview
Tutorial 12.9 introduced the basic INLA framework as well as a fully fleshed out example using a fabricated data set. The current tutorial will focus on linear models and using both fabricated data and real worked examples.
In this tutorial we have an opportunity to explore INLA for multilevel (hierarchical) models.Although performing very simple linear regression with INLA might be seen as a complete overkill (as it does not really utilize the main features for which INLA is designed), from a pedagogical perspective, starting with simpler models for which robust and well understood solutions exist allows us to gradually build up an understanding of how INLA works. Model complexity can be gradually introduced, thereby allowing us to explore techniques progressively by building on more solid foundations. All data sets will be fabricated from set parameters so that we always have a 'truth' from which to compare outcomes and as a point of comparison, each data set will be followed by Frequentist and Bayesian MCMC outcomes.
Simple regression
library(R2jags) library(INLA) library(ggplot2)
Lets say we had set up an experiment in which we applied a continuous treatment ($x$) ranging in magnitude from 0 to 16 to a total of 16 sampling units ($n=16$) and then measured a response ($y$) from each unit. As this section is mainly about the generation of artificial data (and not specifically about what to do with the data), understanding the actual details are optional and can be safely skipped. Consequently, I have folded (toggled) this section away.
- the sample size = 16
- the continuous $x$ variable ranging from 0 to 16
- when the value of $x$ is 0, $y$ is expected to be 40 ($\beta_0=40$)
- a 1 unit increase in $x$ is associated with a 1.5 unit decline in $y$ ($\beta_1=-1.5$)
- the data are drawn from normal distributions with a mean of 0 and standard deviation of 5 ($\sigma^2=25$)
set.seed(1) n <- 16 a <- 40 #intercept b <- -1.5 #slope sigma2 <- 25 #residual variance (sd=5) x <- 1:n #values of the year covariate eps <- rnorm(n, mean = 0, sd = sqrt(sigma2)) #residuals y <- a + b * x + eps #response variable #OR y <- (model.matrix(~x) %*% c(a,b))+eps data <- data.frame(y=round(y,1), x) #dataset head(data) #print out the first six rows of the data set
y x 1 35.4 1 2 37.9 2 3 31.3 3 4 42.0 4 5 34.1 5 6 26.9 6
ggplot(data, aes(y=y, x=x)) + geom_point() + theme_classic()
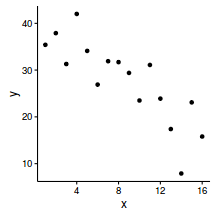
$$ y_i = \alpha + \beta x_i + \varepsilon_i \hspace{4em}\varepsilon_i\sim{}\mathcal{N}(0, \sigma^2) $$
summary(lm(y~x, data=data))
Call:
lm(formula = y ~ x, data = data)
Residuals:
Min 1Q Median 3Q Max
-11.3694 -3.5053 0.6239 2.6522 7.3909
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 40.7450 2.6718 15.250 4.09e-10 ***
x -1.5340 0.2763 -5.552 7.13e-05 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 5.095 on 14 degrees of freedom
Multiple R-squared: 0.6876, Adjusted R-squared: 0.6653
F-statistic: 30.82 on 1 and 14 DF, p-value: 7.134e-05
confint(lm(y~x, data=data))
2.5 % 97.5 % (Intercept) 35.014624 46.4753756 x -2.126592 -0.9413493
modelString=" model { #Likelihood for (i in 1:n) { y[i]~dnorm(mu[i],tau) mu[i] <- alpha+beta*x[i] } #Priors alpha ~ dnorm(0,1.0E-6) beta ~ dnorm(0,1.0E-6) tau <- 1 / (sigma * sigma) sigma~dgamma(0.1,0.001) #tau <- exp(log.tau) #log.tau ~ dgamma(1,0.01) } " data.list <- with(data, list(y=y, x=x, n=nrow(data))) data.jags <- jags(data=data.list, inits=NULL, parameters.to.save=c('alpha','beta', 'sigma'), model.file=textConnection(modelString), n.chains=3, n.iter=100000, n.burnin=20000, n.thin=100 )
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 75 Initializing model
print(data.jags)
Inference for Bugs model at "5", fit using jags,
3 chains, each with 1e+05 iterations (first 20000 discarded), n.thin = 100
n.sims = 2400 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
alpha 40.813 2.837 35.375 38.950 40.766 42.656 46.533 1.001 2400
beta -1.540 0.291 -2.119 -1.729 -1.536 -1.344 -0.973 1.001 2400
sigma 5.383 1.089 3.739 4.600 5.195 6.001 7.939 1.000 2400
deviance 98.660 2.585 95.609 96.706 98.040 99.958 105.200 1.001 2300
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 3.3 and DIC = 102.0
DIC is an estimate of expected predictive error (lower deviance is better).
data.inla <- inla(y~x, data=data, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE))
The inla() function returns an object of class inla of which there are two methods available (summary and plot). For an inla fitted model, the summary method returns the Fixed and Random effects as well as a number of information criterion and limited fit diagnostics.
summary(data.inla)
Call:
c("inla(formula = y ~ x, data = data, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE))")
Time used:
Pre-processing Running inla Post-processing Total
0.1013 0.0632 0.0360 0.2006
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 40.7441 2.5688 35.6573 40.7440 45.8219 40.7442 0
x -1.5339 0.2657 -2.0599 -1.5339 -1.0087 -1.5339 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for the Gaussian observations 0.0438 0.0155 0.0199 0.0418 0.0798 0.0378
Expected number of effective parameters(std dev): 2.00(0.00)
Number of equivalent replicates : 8.00
Deviance Information Criterion (DIC) ...: 100.64
Effective number of parameters .........: 2.634
Watanabe-Akaike information criterion (WAIC) ...: 101.40
Effective number of parameters .................: 2.942
Marginal log-Likelihood: -64.55
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
Lets focus on the different components of this output. The 'Fixed' effects represent a set of summaries from the posterior distribution. These results are very similar to those yielded from the Frequentist and Bayesian MCMC approaches.
mean sd 0.025quant 0.5quant 0.975quant mode kld (Intercept) 40.7441 2.5688 35.6573 40.7440 45.8219 40.7442 0 x -1.5339 0.2657 -2.0599 -1.5339 -1.0087 -1.5339 0
mean sd 0.025quant 0.5quant 0.975quant mode Precision for the Gaussian observations 0.0438 0.0155 0.0199 0.0418 0.0798 0.0378
Expected number of effective parameters(std dev): 2.00(0.00) Number of equivalent replicates : 8.00
The number of equivalent replicates is the number of replicates (sample size) per effective parameter. In this case, there are 16 observations, so there are $16/2=8$ equivalent replicates. Low values (relative to the overall sample size) are indicative of a poor fit.
Out-of-sample or leave-one-out predictive performance
Arguably, the best way to assess the fit of a model is via an estimate of the models predictive accuracy, the gold standard of which is out-of-sample estimation via cross-validation (in which each observed response is compared to a prediction derived from a model that trained on without the focal response value). However, as this approach required repeated model fits, it is not overly practical for large complex models, MCMC simulations or very sparse data. Traditional alternatives to cross-validation include Akaike's Information Criterion (AIC) and Deviance Information Criterion (DIC) when associated with Bayesian analyses.
Deviance Information Criterion (DIC)...: 100.64 Effective number of parameters .........: 2.634
Watanabe-Akaike information criterion (WAIC) ...: 101.40 Effective number of parameters .................: 2.942
The effective number of parameters (pD) is the posterior mean deviance minus the deviance measured at the posterior mean of the parameters. Both the DIC and pD are similar to those reported by JAGS.
Wantanabe-Akaike Information Criterion or Widely Applicable Information Criterion (WAIC). By contrast to AIC (and DIC) WAIC is a more fully Bayesian approach for estimating the out-of-sample expectation based on the log pointwise posterior predictive density.
Two additional out-of-sample estimates (measures of fit) supported by INLA include:
- Conditional Predictive Ordinate (CPO) is the density of the observed value of $y_i$ within the out-of-sample ($y_{-i}$) posterior predictive distribution.
A small CPO value associated with an observation suggests that this observation is unlikely (or surprising) in light of the model, priors and other
data in the model. In addition, the sum of the CPO values (or alternatively, the negative of the mean natural logarithm of the CPO values) is a measure of fit.
In this case there are no CPO values that are considerably smaller (order of magnitude smaller) than the others - so with respect to the model, none of the observed values would be considered 'surprising'. Various assumptions are made behind INLA's computations. If any of these assumptions fail of an observation, it is flagged within (in this case) cpo$failure as a non-zero. When this is the case, it is prudent to recalculate the CPO values after removing the observations associated with failure flags not equal to zero and re-fitting the model. This can be performed using the inla.cpo() function.
data.inla$cpo$cpo
[1] 0.047749222 0.074109301 0.041001671 0.018540996 0.076175384 0.046941565 0.073009041 0.062423313 [9] 0.069163109 0.072927701 0.021584385 0.073660042 0.057706132 0.001797082 0.033613593 0.071977093
sum(data.inla$cpo$cpo)
[1] 0.8423796
-mean(log(data.inla$cpo$cpo))
[1] 3.173897
In this case, there are no non-zero CPO values.data.inla$cpo$failure
[1] 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
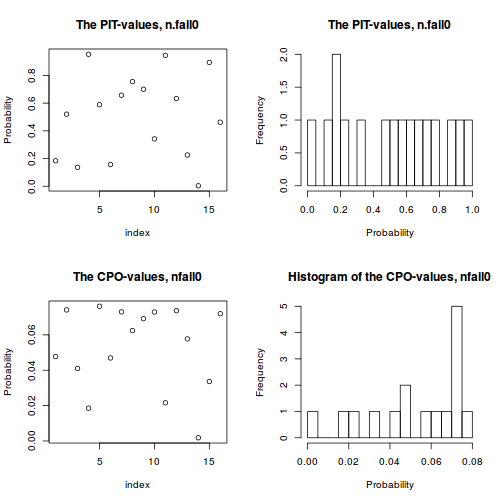
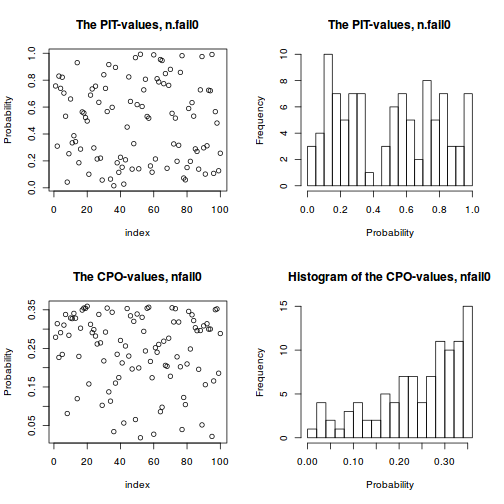
- Probability Integral Transforms (PIT) provides a version of CPO that is calibrated to the level of the Gaussian field so that it is clearer
whether or not any of the values are 'small' (all values must be between 0 and 1). A histogram of PIT values that does not look approximately uniform (flat) indicates a lack of model fit.
In this case the PIT values do not appear to deviate from a uniform distribution and thus do not indicate a lack of fit.
plot(data.inla, plot.fixed.effects=FALSE, plot.lincomb=FALSE, plot.random.effects=FALSE, plot.hyperparameters=FALSE, plot.predictor=FALSE, plot.q=FALSE, plot.cpo=TRUE, single=FALSE)
The influence of priors
When fitting the INLA model above, we did not specify priors. Consequently, the default priors were employed. To review what these priors are, we can use the inla.show.hyperspec() function - which returns a list containing information about all the hyper-priors used in a model.
inla.show.hyperspec(data.inla)
List of 3
$ predictor:List of 1
$ hyper:List of 1
$ theta:List of 8
$ name : atomic [1:1] log precision
$ short.name: atomic [1:1] prec
$ initial : atomic [1:1] 11
$ fixed : atomic [1:1] TRUE
$ prior : atomic [1:1] loggamma
$ param : atomic [1:2] 1e+00 1e-05
$ family :List of 1
$ :List of 3
$ label: chr "gaussian"
$ hyper:List of 1
$ theta:List of 8
$ name : atomic [1:1] log precision
$ short.name: atomic [1:1] prec
$ initial : atomic [1:1] 4
$ fixed : atomic [1:1] FALSE
$ prior : atomic [1:1] loggamma
$ param : atomic [1:2] 1e+00 5e-05
$ link :List of 1
$ hyper: list()
$ fixed :List of 2
$ :List of 3
$ label : chr "(Intercept)"
$ prior.mean: num 0
$ prior.prec: num 0
$ :List of 3
$ label : chr "x"
$ prior.mean: num 0
$ prior.prec: num 0.001
In order to drive the specification of weekly informative priors for variance components (a prior that allows the data to dictate the parameter estimates whilst constraining the range of the marginal distribution within sensible bounds), we can consider the range over which the marginal distribution of variance components would be sensible. For the range [-R,R], the equivalent gamma parameters: $$ \begin{align} a &= \frac{d}{2}\\ b &= \frac{R^2d}{2*(t^d_{1-(1-q)/2})^2} \end{align} $$ where $d$ is the degrees of freedom (for Cauchy marginal, $d=1$) and $t^d_{1-(1-q)/2}$ is the $q$th quantile of a Student $t$ with $d$ degrees of freedom. Hence if we considered variance likely to be in the range of [0,10], we could define a loggamma prior of: $$ \begin{align} a &= \frac{1}{2} = 0.5\\ b &= \frac{10^2}{2*161.447} = 0.31\\ \tau &\sim{} log\Gamma(0.5,0.31) \end{align} $$
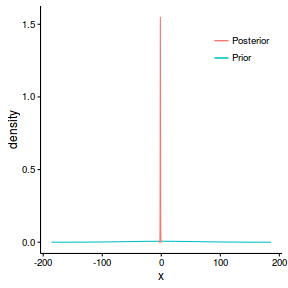
The priors for the fixed effects (intercept and slope) are both Normal distributions
with mean of 0 and precision (0.001). This implies that the prior for slope has a standard deviation
of approximately 31 (since $\sigma = \frac{1}{\sqrt{\tau}}$). As a general rule, three standard deviations
envelopes most of a distribution, and thus this prior defines a distribution whose density is almost entirely
within the range [-93,93]. Whilst this might be appropriate
for the intercept, it is arguably very wide for the slope given the range of the predictor variable.
In order to generate realistic informative Gaussian priors (for the purpose of constraining the posterior to a logical range) for fixed parameters, the following formulae are useful: $$ \begin{align} \mu &= \frac{z_2\theta_1 - z_1\theta_2}{z_2-z_1}\\ \sigma &= \frac{\theta_2 - \theta_1}{z_2-z_1} \end{align} $$ where $\theta_1$ and $\theta_2$ are the quantiles on the response scale and $z_1$ and $z_2$ are the corresponding quantiles on the standard normal scale. Hence, if we considered that the slope is likely to be in the range of [-10,10], we could specify a Normal prior with mean of ($\mu=\frac{(qnorm(0.5,0,1)*0) - (qnorm(0.975,0,1)*10)}{10-0} = 0$) and a standard deviation of ($\mu=\frac{10 - 0}{qnorm(0.975,0,1)-qnorm(0.5,0,1)} = 5.102$). In INLA (which defines priors in terms of precision rather than standard deviation), the associated prior would be $\beta \sim{} \mathcal{N}(0, 0.196)$.
Lets plot the prior and posterior for slope.
#the default priors specify a standard deviation of: (SD<-(1/sqrt(0.001) * qnorm(0.975,0,1)))
[1] 61.9795
prior <- data.frame(x=seq(-3*SD,3*SD,len=1000)) prior$density <- dnorm(prior$x,0,SD) post <- data.frame(data.inla$marginals.fixed[[2]]) head(post)
x y 1 -4.190463 1.765936e-16 2 -3.659143 9.807886e-11 3 -3.127823 1.422271e-06 4 -2.862163 6.240289e-05 5 -2.596503 1.483329e-03 6 -2.330843 2.134545e-02
ggplot(prior, aes(y=density, x=x)) + geom_line(aes(color='Prior')) + geom_line(data=post, aes(y=y, x=x, color='Posterior')) + scale_color_discrete('')+ theme_classic()+theme(legend.position=c(1,1), legend.justification=c(1,1))
s <- inla.contrib.sd(data.inla, nsamples = 1000) s$hyper
mean sd 2.5% 97.5% sd for the Gaussian observations 4.991154 0.9202552 3.527553 7.121831
data.inla <- inla(y~x, data=data, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE), control.fixed=list(mean=0, prec=0.196, mean.intercept=0, prec.intercept=0.001), control.family=list(hyper=list(prec=list(prior='loggamma', param=c(0.1,0.1))))) summary(data.inla)
Call:
c("inla(formula = y ~ x, data = data, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE), control.family = list(hyper = list(prec = list(prior = \"loggamma\", ", " param = c(0.1, 0.1)))), control.fixed = list(mean = 0, prec = 0.196, ", " mean.intercept = 0, prec.intercept = 0.001))")
Time used:
Pre-processing Running inla Post-processing Total
0.0911 0.0326 0.0285 0.1523
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 40.2459 2.7140 34.8045 40.2675 45.5553 40.3057 0
x -1.4833 0.2804 -2.0322 -1.4855 -0.9215 -1.4894 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for the Gaussian observations 0.0388 0.0146 0.0167 0.0368 0.073 0.0328
Expected number of effective parameters(std dev): 1.977(0.0069)
Number of equivalent replicates : 8.092
Deviance Information Criterion (DIC) ...: 100.79
Effective number of parameters .........: 2.636
Watanabe-Akaike information criterion (WAIC) ...: 101.14
Effective number of parameters .................: 2.604
Marginal log-Likelihood: -56.99
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
data.inla <- inla(y~x, data=data, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE), control.fixed=list(mean=0, prec=0.0196, mean.intercept=0, prec.intercept=0.001), control.family=list(hyper=list(prec=list(prior='loggamma', param=c(0.1,0.1))))) summary(data.inla)
Call:
c("inla(formula = y ~ x, data = data, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE), control.family = list(hyper = list(prec = list(prior = \"loggamma\", ", " param = c(0.1, 0.1)))), control.fixed = list(mean = 0, prec = 0.0196, ", " mean.intercept = 0, prec.intercept = 0.001))")
Time used:
Pre-processing Running inla Post-processing Total
0.0923 0.0239 0.0286 0.1448
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 40.4222 2.7267 34.9757 40.4364 45.7778 40.4616 0
x -1.5041 0.2822 -2.0593 -1.5054 -0.9414 -1.5077 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for the Gaussian observations 0.0388 0.0146 0.0167 0.0368 0.073 0.0328
Expected number of effective parameters(std dev): 1.991(0.0028)
Number of equivalent replicates : 8.036
Deviance Information Criterion (DIC) ...: 100.80
Effective number of parameters .........: 2.651
Watanabe-Akaike information criterion (WAIC) ...: 101.16
Effective number of parameters .................: 2.62
Marginal log-Likelihood: -57.94
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
s <- inla.contrib.sd(data.inla, nsamples = 1000) s$hyper
mean sd 2.5% 97.5% sd for the Gaussian observations 5.337874 1.088706 3.663546 7.863846
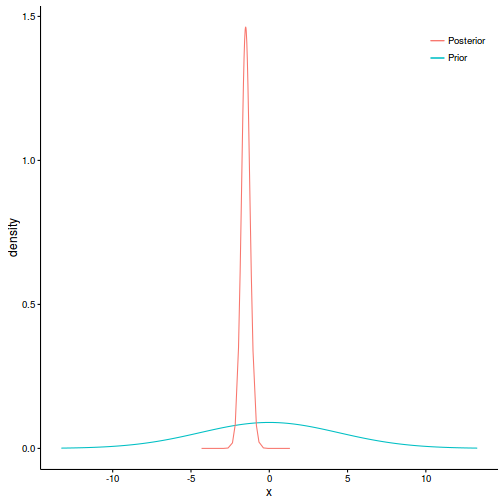
#the default priors specify a standard deviation of: (SD<-(1/sqrt(0.196) * qnorm(0.975,0,1)))
[1] 4.427107
prior <- data.frame(x=seq(-3*SD,3*SD,len=1000)) prior$density <- dnorm(prior$x,0,SD) post <- data.frame(data.inla$marginals.fixed[[2]]) head(post)
x y 1 -4.325697 2.134404e-16 2 -3.761375 1.037706e-10 3 -3.197053 1.349437e-06 4 -2.914892 5.713188e-05 5 -2.632731 1.331189e-03 6 -2.350570 1.925008e-02
ggplot(prior, aes(y=density, x=x)) + geom_line(aes(color='Prior')) + geom_line(data=post, aes(y=y, x=x, color='Posterior')) + scale_color_discrete('')+ theme_classic()+theme(legend.position=c(1,1), legend.justification=c(1,1))
More detailed information
In addition to the PIT and CPO distributions, there are numerous other summary plots available, each of which summarizes the marginal distribution of the different types of model parameters (fixed, random, hyper-parameters etc) and predictions. These can be produced in one hit using the plot() function. However, I will produce them individually in order to assist discussion. Moreover, as a result of complexities arising from the need for the plot() function to work in Rstudio, INLA's plot() function and knitr are slightly incompatible at the moment...
Additionally, the fitted INLA object (a list) contains a large number of items, each of which capture different aspects of the approximated parameters.
names(data.inla)
[1] "names.fixed" "summary.fixed" "marginals.fixed" [4] "summary.lincomb" "marginals.lincomb" "size.lincomb" [7] "summary.lincomb.derived" "marginals.lincomb.derived" "size.lincomb.derived" [10] "mlik" "cpo" "po" [13] "waic" "model.random" "summary.random" [16] "marginals.random" "size.random" "summary.linear.predictor" [19] "marginals.linear.predictor" "summary.fitted.values" "marginals.fitted.values" [22] "size.linear.predictor" "summary.hyperpar" "marginals.hyperpar" [25] "internal.summary.hyperpar" "internal.marginals.hyperpar" "offset.linear.predictor" [28] "model.spde2.blc" "summary.spde2.blc" "marginals.spde2.blc" [31] "size.spde2.blc" "model.spde3.blc" "summary.spde3.blc" [34] "marginals.spde3.blc" "size.spde3.blc" "logfile" [37] "misc" "dic" "mode" [40] "neffp" "joint.hyper" "nhyper" [43] "version" "Q" "graph" [46] "ok" "cpu.used" "all.hyper" [49] ".args" "call" "model.matrix"
This is obviously a very large list. In order to make more sense of all that is available, we can group the returned INLA list into functional categories:
- parameter estimates
INLA object Description names.fixed the names of fixed effects parameters summary.fixed summaries (mean, standard deviation and quantiles) of fixed posteriors (on link scale) marginals.fixed list of posterior marginal densities of the fixed effects size.random number of levels of each of the structured random covariates summary.random summaries (mean, standard deviation and quantiles) of the structured random effects (smoothers etc) marginals.random list of posterior marginal densities of the random effects summary.hyperpar summaries (mean, standard deviation and quantiles) of the model hyper-parameters (precision, overdispersion, correlation etc) marginals.hyperpar list of posterior marginal densities of the model hyper-parameters - predictions and contrasts
INLA object Description model.matrix model matrix of the fixed effects - a row for each row of the input data summary.linear.predictor summaries (mean, standard deviation and quantiles) of the linear predictor - a prediction (on the link scale) associated with each row of the input data marginals.linear.predictor list of posterior marginal densities of the linear predictor summary.fitted.values summaries (mean, standard deviation and quantiles) of the fitted values - a prediction (on the response scale) associated with each row of the input data marginals.fitted.values list of posterior marginal densities of the fitted values summary.lincomb summaries (mean, standard deviation and quantiles) of the defined linear combinations (on the link scale) marginals.lincomb list of posterior marginal densities of the defined linear combinations summary.lincomb.derived summaries (mean, standard deviation and quantiles) of the defined linear combinations (on the response scale) marginals.lincomb.derived list of posterior marginal densities of the defined linear combinations (response scale) - model performance measures
INLA object Description mlik log marginal likelihood of the model (when mlik=TRUE) dic deviance information criterion of the model (when dic=TRUE) waic Watanabe-Akaike (Widely Applicable) information criterion of the model (when dic=TRUE) neffp expected number of parameters (and standard deviation) and number of replicates per parameter in the model cpo conditional predictive ordinate (cpo), probability integral transform (pit) and vector of assumption failures (failures) of the model (when cpo=TRUE)
Fixed effects
plot(data.inla, plot.fixed.effects = TRUE, plot.lincomb = FALSE, plot.random.effects = FALSE, plot.hyperparameters = FALSE, plot.predictor = FALSE, plot.q = FALSE, plot.cpo = FALSE, single = FALSE)
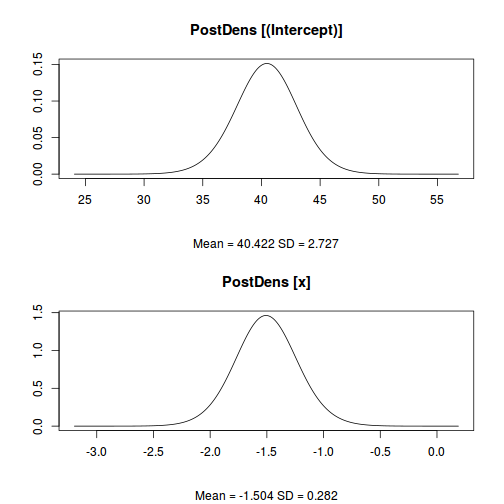
data.inla$summary.fixed
mean sd 0.025quant 0.5quant 0.975quant mode kld (Intercept) 40.422174 2.7267362 34.97573 40.436435 45.7778033 40.461613 3.011495e-11 x -1.504087 0.2821624 -2.05931 -1.505424 -0.9413974 -1.507741 2.972068e-11
data.inla$marginals.fixed
$`(Intercept)`
x y
[1,] 13.15496 4.410151e-17
[2,] 18.60840 1.905779e-11
[3,] 24.06185 2.152067e-07
[4,] 26.78857 8.435060e-06
[5,] 29.51529 1.784278e-04
[6,] 32.24201 2.276125e-03
[7,] 33.83266 8.280310e-03
[8,] 35.92316 3.499961e-02
[9,] 36.96775 6.200022e-02
[10,] 37.65244 8.427154e-02
[11,] 38.19117 1.028661e-01
[12,] 38.64056 1.178672e-01
[13,] 39.04390 1.299800e-01
[14,] 39.41565 1.392874e-01
[15,] 39.76649 1.459042e-01
[16,] 40.10455 1.499193e-01
[17,] 40.27099 1.509687e-01
[18,] 40.43643 1.513822e-01
[19,] 40.60209 1.511608e-01
[20,] 40.76790 1.503044e-01
[21,] 41.10458 1.466640e-01
[22,] 41.45260 1.404086e-01
[23,] 41.81934 1.314582e-01
[24,] 42.21872 1.195765e-01
[25,] 42.66654 1.045571e-01
[26,] 43.18698 8.627934e-02
[27,] 43.85476 6.385703e-02
[28,] 44.86945 3.628873e-02
[29,] 46.88034 8.629793e-03
[30,] 48.60235 1.975121e-03
[31,] 51.32907 1.352744e-04
[32,] 54.05579 5.756324e-06
[33,] 56.78251 1.349249e-07
[34,] 62.23596 1.021137e-11
[35,] 67.68940 2.068547e-17
$x
x y
[1,] -4.32569687 2.134404e-16
[2,] -3.76137496 1.037706e-10
[3,] -3.19705306 1.349437e-06
[4,] -2.91489210 5.713188e-05
[5,] -2.63273115 1.331189e-03
[6,] -2.35057020 1.925008e-02
[7,] -2.17334332 8.304301e-02
[8,] -1.96491059 3.492400e-01
[9,] -1.85981324 6.148759e-01
[10,] -1.79056620 8.315525e-01
[11,] -1.73580056 1.011021e+00
[12,] -1.69001537 1.154394e+00
[13,] -1.64879258 1.269090e+00
[14,] -1.61067721 1.356196e+00
[15,] -1.57459777 1.416978e+00
[16,] -1.53973520 1.452399e+00
[17,] -1.52252620 1.460799e+00
[18,] -1.50542406 1.463031e+00
[19,] -1.48827869 1.459127e+00
[20,] -1.47107359 1.449088e+00
[21,] -1.43609861 1.410468e+00
[22,] -1.39985194 1.346777e+00
[23,] -1.36155020 1.257371e+00
[24,] -1.31985206 1.140529e+00
[25,] -1.27276242 9.936500e-01
[26,] -1.21787798 8.165285e-01
[27,] -1.14702386 6.008029e-01
[28,] -1.03864956 3.384795e-01
[29,] -0.82220815 7.976568e-02
[30,] -0.65760448 2.185504e-02
[31,] -0.37544353 1.705125e-03
[32,] -0.09328257 8.038838e-05
[33,] 0.18887838 2.048111e-06
[34,] 0.75320028 1.812337e-10
[35,] 1.31752219 4.198486e-16
library(dplyr) data.inla.fixed <- reshape2:::melt(data.inla$marginals.fixed) %>% reshape2:::dcast(L1+Var1~Var2, value='value') ggplot(data.inla.fixed, aes(y=y, x=x)) + geom_line() + facet_wrap(~L1, scales='free', nrow=1) + theme_classic()
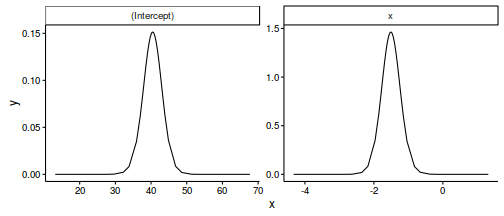
Predictor
plot(data.inla, plot.fixed.effects=FALSE, plot.lincomb=FALSE, plot.random.effects=FALSE, plot.hyperparameters=FALSE, plot.predictor=TRUE, plot.q=FALSE, plot.cpo=FALSE, single=FALSE)
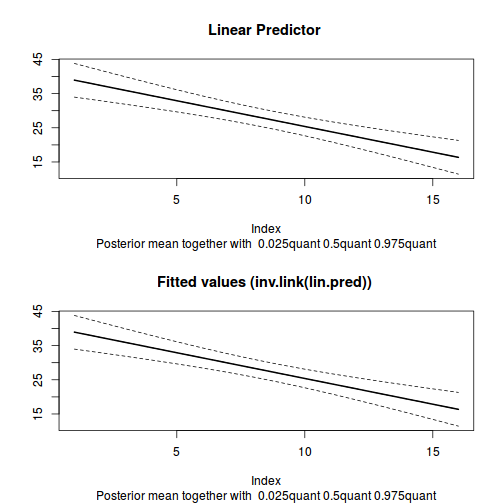
data.inla$summary.linear.predictor
mean sd 0.025quant 0.5quant 0.975quant mode kld predictor.01 38.91874 2.483244 33.96448 38.93146 43.79792 38.95390 1.007436e-07 predictor.02 37.41459 2.247989 32.92995 37.42602 41.83181 37.44617 1.014385e-07 predictor.03 35.91045 2.024777 31.87159 35.92057 39.88955 35.93843 1.027624e-07 predictor.04 34.40632 1.818051 30.78060 34.41514 37.97993 34.43071 1.051053e-07 predictor.05 32.90219 1.634077 29.64458 32.90971 36.11533 32.92298 1.090693e-07 predictor.06 31.39807 1.481359 28.44673 31.40428 34.31258 31.41524 1.154613e-07 predictor.07 29.89397 1.370386 27.16634 29.89887 32.59239 29.90751 1.250763e-07 predictor.08 28.38989 1.311798 25.78241 28.39345 30.97613 28.39978 1.380414e-07 predictor.09 26.88582 1.312630 24.28112 26.88804 29.47847 26.89203 1.530650e-07 predictor.10 25.38177 1.372775 22.66255 25.38262 28.09812 25.38426 1.678379e-07 predictor.11 23.87775 1.485046 20.93976 23.87723 26.82085 23.87651 1.806989e-07 predictor.12 22.37373 1.638769 19.13512 22.37184 25.62556 22.36874 1.923988e-07 predictor.13 20.86972 1.823489 17.26929 20.86646 24.49158 20.86098 2.011791e-07 predictor.14 19.36572 2.030767 15.35897 19.36108 23.40225 19.35322 2.077685e-07 predictor.15 17.86174 2.254388 13.41636 17.85572 22.34529 17.84548 2.126772e-07 predictor.16 16.35775 2.489953 11.45012 16.35035 21.31196 16.33773 2.163935e-07
data.inla$summary.fitted.values
mean sd 0.025quant 0.5quant 0.975quant mode fitted.predictor.01 38.91874 2.483244 33.96448 38.93146 43.79792 38.95390 fitted.predictor.02 37.41459 2.247989 32.92995 37.42602 41.83181 37.44617 fitted.predictor.03 35.91045 2.024777 31.87159 35.92057 39.88955 35.93843 fitted.predictor.04 34.40632 1.818051 30.78060 34.41514 37.97993 34.43071 fitted.predictor.05 32.90219 1.634077 29.64458 32.90971 36.11533 32.92298 fitted.predictor.06 31.39807 1.481359 28.44673 31.40428 34.31258 31.41524 fitted.predictor.07 29.89397 1.370386 27.16634 29.89887 32.59239 29.90751 fitted.predictor.08 28.38989 1.311798 25.78241 28.39345 30.97613 28.39978 fitted.predictor.09 26.88582 1.312630 24.28112 26.88804 29.47847 26.89203 fitted.predictor.10 25.38177 1.372775 22.66255 25.38262 28.09812 25.38426 fitted.predictor.11 23.87775 1.485046 20.93976 23.87723 26.82085 23.87651 fitted.predictor.12 22.37373 1.638769 19.13512 22.37184 25.62556 22.36874 fitted.predictor.13 20.86972 1.823489 17.26929 20.86646 24.49158 20.86098 fitted.predictor.14 19.36572 2.030767 15.35897 19.36108 23.40225 19.35322 fitted.predictor.15 17.86174 2.254388 13.41636 17.85572 22.34529 17.84548 fitted.predictor.16 16.35775 2.489953 11.45012 16.35035 21.31196 16.33773
Hyper-parameters
plot(data.inla, plot.fixed.effects=FALSE, plot.lincomb=FALSE, plot.random.effects=FALSE, plot.hyperparameters=TRUE, plot.predictor=FALSE, plot.q=FALSE, plot.cpo=FALSE, single=FALSE)
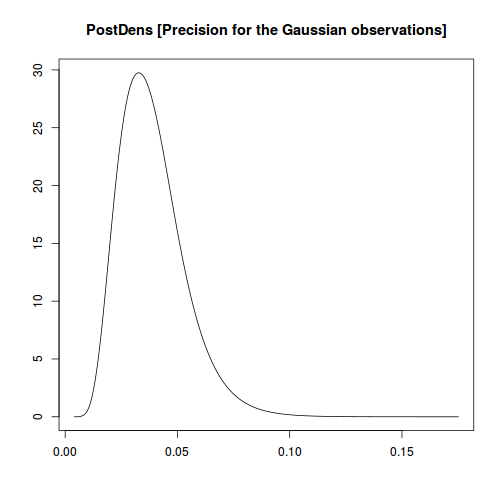
data.inla$summary.hyperpar
mean sd 0.025quant 0.5quant 0.975quant mode Precision for the Gaussian observations 0.03882442 0.01458731 0.01670641 0.03679713 0.07303003 0.03278234
s <- inla.contrib.sd(data.inla, nsamples = 1000) s$hyper
mean sd 2.5% 97.5% sd for the Gaussian observations 5.393922 0.9865159 3.717143 7.535196
5.394 which is similar to the JAGS estimate.
Predictions
There are two main ways to generate predictions in INLA:
- append additional data cases with missing (NA) response to the model input data. Missing response data will not contribute to the likelihood, yet will
be imputed (estimated/predicted given the model trends). In order to illustrate producing a summary plot, I will predict the response for a sequence of predictor
values.
newdata <- data.frame(x=seq(min(data$x, na.rm=TRUE), max(data$x, na.rm=TRUE), len=100), y=NA) data.pred <- rbind(data, newdata) #fit the model data.inla1 <- inla(y~x, data=data.pred, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE), control.predictor=list(compute=TRUE)) #examine the regular summary - not there are no changes to the first fit summary(data.inla1)
Call: c("inla(formula = y ~ x, data = data.pred, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE), control.predictor = list(compute = TRUE))" ) Time used: Pre-processing Running inla Post-processing Total 0.0663 0.0489 0.0239 0.1391 Fixed effects: mean sd 0.025quant 0.5quant 0.975quant mode kld (Intercept) 40.7441 2.5688 35.6573 40.7440 45.8219 40.7442 0 x -1.5339 0.2657 -2.0599 -1.5339 -1.0087 -1.5339 0 The model has no random effects Model hyperparameters: mean sd 0.025quant 0.5quant 0.975quant mode Precision for the Gaussian observations 0.0438 0.0155 0.0199 0.0418 0.0798 0.0378 Expected number of effective parameters(std dev): 2.00(0.00) Number of equivalent replicates : 8.00 Deviance Information Criterion (DIC) ...: 100.64 Effective number of parameters .........: 2.634 Watanabe-Akaike information criterion (WAIC) ...: 101.40 Effective number of parameters .................: 2.942 Marginal log-Likelihood: -64.55 CPO and PIT are computed Posterior marginals for linear predictor and fitted values computed#extract the predictor values associated with the appended data newdata <- cbind(newdata, data.inla1$summary.linear.predictor[(nrow(data)+1):nrow(data.pred),]) head(newdata)
x y mean sd 0.025quant 0.5quant 0.975quant mode kld predictor.017 1.000000 NA 39.21085 2.339342 34.58500 39.21057 43.83805 39.21031 1.031930e-07 predictor.018 1.151515 NA 38.97844 2.305138 34.42022 38.97816 43.53798 38.97790 1.031943e-07 predictor.019 1.303030 NA 38.74603 2.271133 34.25505 38.74575 43.23831 38.74550 1.031957e-07 predictor.020 1.454545 NA 38.51362 2.237335 34.08947 38.51334 42.93905 38.51309 1.031972e-07 predictor.021 1.606061 NA 38.28120 2.203755 33.92346 38.28094 42.64021 38.28069 1.031987e-07 predictor.022 1.757576 NA 38.04879 2.170402 33.75700 38.04853 42.34183 38.04828 1.032003e-07
newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) ggplot(newdata, aes(y=mean, x=x)) + geom_point(data=data, aes(y=y)) + geom_ribbon(aes(ymin=lower, ymax=upper), fill='blue', alpha=0.2) + geom_line() + theme_classic()
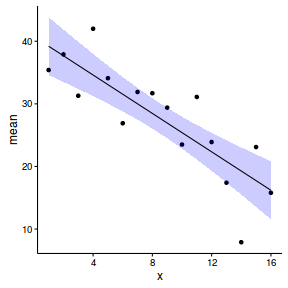
- define linear combinations (contrasts) for the predictions. Note, the inla.make.lincombs() function seems a little fragile.
It is vital that it be parsed a data.frame. Furthermore, the model matrix needs to be converted to a data frame using the as.data.frame()
function rather than the data.frame() function as the latter alters the column names and causes problems within INLA.
newdata <- data.frame(x=seq(min(data$x, na.rm=TRUE), max(data$x, na.rm=TRUE), len=100)) Xmat <- model.matrix(~x, data=newdata) lincomb <- inla.make.lincombs(as.data.frame(Xmat)) #fit the model data.inla1 <- inla(y~x, lincomb=lincomb, #include the linear combinations data=data, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE), control.inla=list(lincomb.derived.only=FALSE)) #examine the regular summary - not there are no changes to the first fit #summary(data.inla1) head(data.inla1$summary.lincomb)
ID mean sd 0.025quant 0.5quant 0.975quant mode kld lc001 1 39.20315 2.359954 34.52673 39.20440 43.86357 39.20635 1.371747e-11 lc002 2 38.97090 2.325452 34.36286 38.97213 43.56320 38.97404 1.372809e-11 lc003 3 38.73864 2.291151 34.19858 38.73985 43.26322 38.74173 1.370907e-11 lc004 4 38.50639 2.257060 34.03389 38.50758 42.96365 38.50942 1.371347e-11 lc005 5 38.27414 2.223188 33.86877 38.27530 42.66452 38.27711 1.373200e-11 lc006 6 38.04188 2.189545 33.70319 38.04303 42.36584 38.04480 1.369481e-11
newdata <- cbind(newdata, data.inla1$summary.lincomb) newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) ggplot(newdata, aes(y=mean, x=x)) + geom_point(data=data, aes(y=y)) + geom_ribbon(aes(ymin=lower, ymax=upper), fill='blue', alpha=0.2) + geom_line() + theme_classic()
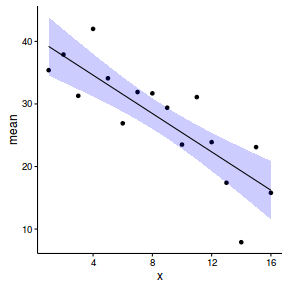
One very good way to evaluate the fit of a model is on how well it estimates known parameters. This example generated simulated data given a set of parameters. We could therefore compare the model parameter estimates to these truths.
orig <- data.frame(parameter=factor(c('(Intercept)','x','Sigma2')), value=c(40,-1.5,5)) mod <- subset(data.inla$summary.fixed, select=c('mean','sd','0.025quant','0.975quant')) mod <- reshape::rename(mod,c("0.025quant"="lower", "0.975quant"="upper")) colnames(s$hyper) <- c('mean','sd','lower','upper') mod <- rbind(mod, s$hyper) mod$parameter <- factor(c('(Intercept)','x','Sigma2')) ggplot(mod, aes(x=parameter, y=mean)) + geom_hline(yintercept=0, linetype='dashed')+ geom_blank()+ geom_linerange(aes(x=as.numeric(parameter)-0.05,ymin=lower, ymax=upper)) + geom_point(data=orig, aes(y=value, x=as.numeric(factor(parameter))+0.05, fill='Original'), shape=21)+ geom_point(aes(x=as.numeric(parameter)-0.05, fill='Model'), shape=21)+ coord_flip()+ scale_fill_discrete('')+ scale_y_continuous('')+ theme_classic()+theme(legend.position=c(1,1), legend.justification=c(1,1))
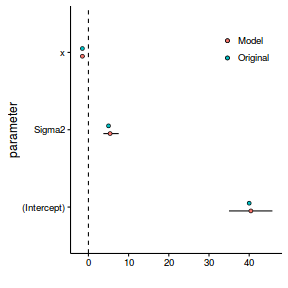
Hence, it appears that the estimated (modelled) parameters are entirely consistent with the original parameters from which the data were simulated.
Multiple linear regression
In the previous example, I displayed many of the basic components of the fitted INLA model. In the remaining examples, we will build upon some of these and ignore others completely - depending on what is required...
Lets say we had set up a natural experiment in which we measured a response ($y$) from each of 20 sampling units ($n=20$) across a landscape. At the same time, we also measured two other continuous covariates ($x1$ and $x2$) from each of the sampling units. As this section is mainly about the generation of artificial data (and not specifically about what to do with the data), understanding the actual details are optional and can be safely skipped. Consequently, I have folded (toggled) this section away.
set.seed(3) n = 100 intercept = 5 temp = runif(n) nitro = runif(n) + 0.8*temp int.eff=2 temp.eff <- 0.85 nitro.eff <- 0.5 res = rnorm(n, 0, 1) coef <- c(int.eff, temp.eff, nitro.eff,int.eff) mm <- model.matrix(~temp*nitro) y <- t(coef %*% t(mm)) + res data <- data.frame(y,x1=temp, x2=nitro, cx1=scale(temp,scale=F), cx2=scale(nitro,scale=F))
$$ y_i = \alpha + \beta_1 x_{1i} + \beta_2 x_{2i} + \varepsilon_i \hspace{4em}\varepsilon_i\sim{}\mathcal{N}(0, \sigma^2) $$
#additive model summary(lm(y~cx1+cx2, data=data))
Call:
lm(formula = y ~ cx1 + cx2, data = data)
Residuals:
Min 1Q Median 3Q Max
-2.45927 -0.78738 -0.00688 0.71051 2.88492
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 3.8234 0.1131 33.793 < 2e-16 ***
cx1 3.0250 0.4986 6.067 2.52e-08 ***
cx2 1.3878 0.4281 3.242 0.00163 **
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 1.131 on 97 degrees of freedom
Multiple R-squared: 0.5361, Adjusted R-squared: 0.5265
F-statistic: 56.05 on 2 and 97 DF, p-value: < 2.2e-16
confint(lm(y~cx1+cx2, data=data))
2.5 % 97.5 % (Intercept) 3.5988464 4.047962 cx1 2.0353869 4.014675 cx2 0.5381745 2.237488
#multiplicative model summary(lm(y~cx1*cx2, data=data))
Call:
lm(formula = y ~ cx1 * cx2, data = data)
Residuals:
Min 1Q Median 3Q Max
-2.34877 -0.85435 0.06905 0.71265 2.57068
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 3.6710 0.1315 27.924 < 2e-16 ***
cx1 2.9292 0.4914 5.961 4.15e-08 ***
cx2 1.3445 0.4207 3.196 0.00189 **
cx1:cx2 2.6651 1.2305 2.166 0.03281 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 1.111 on 96 degrees of freedom
Multiple R-squared: 0.5577, Adjusted R-squared: 0.5439
F-statistic: 40.35 on 3 and 96 DF, p-value: < 2.2e-16
confint(lm(y~cx1*cx2, data=data))
2.5 % 97.5 % (Intercept) 3.4100621 3.931972 cx1 1.9537887 3.904642 cx2 0.5094571 2.179451 cx1:cx2 0.2224680 5.107650
#additive model modelString=" model { #Likelihood for (i in 1:n) { y[i]~dnorm(mu[i],tau) mu[i] <- beta0+beta1*x1[i]+beta2*x2[i] } #Priors beta0 ~ dnorm(0.01,1.0E-6) beta1 ~ dnorm(0,1.0E-6) beta2 ~ dnorm(0,1.0E-6) tau <- 1 / (sigma * sigma) sigma~dunif(0,100) } " data.list <- with(data, list(y=y, x1=cx1, x2=cx2, n=nrow(data))) data.jags <- jags(data=data.list, inits=NULL, parameters.to.save=c('alpha','beta1', 'beta2', 'sigma'), model.file=textConnection(modelString), n.chains=3, n.iter=100000, n.burnin=20000, n.thin=100 )
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 612 Initializing model
print(data.jags)
Inference for Bugs model at "6", fit using jags,
3 chains, each with 1e+05 iterations (first 20000 discarded), n.thin = 100
n.sims = 2400 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta1 3.022 0.504 2.013 2.684 3.006 3.360 4.008 1.001 2400
beta2 1.388 0.435 0.498 1.113 1.403 1.689 2.199 1.001 2400
sigma 1.149 0.082 1.002 1.091 1.144 1.202 1.325 1.000 2400
deviance 309.506 2.931 305.926 307.384 308.842 310.830 317.254 1.001 2400
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 4.3 and DIC = 313.8
DIC is an estimate of expected predictive error (lower deviance is better).
#multiplicative model modelString=" model { #Likelihood for (i in 1:n) { y[i]~dnorm(mu[i],tau) mu[i] <- beta0+beta1*x1[i]+beta2*x2[i]+beta3*x1[i]*x2[i] } #Priors beta0 ~ dnorm(0.01,1.0E-6) beta1 ~ dnorm(0,1.0E-6) beta2 ~ dnorm(0,1.0E-6) beta3 ~ dnorm(0,1.0E-6) tau <- 1 / (sigma * sigma) sigma~dunif(0,100) } " data.list <- with(data, list(y=y, x1=cx1, x2=cx2, n=nrow(data))) data.jags <- jags(data=data.list, inits=NULL, parameters.to.save=c('alpha','beta1', 'beta2', 'sigma'), model.file=textConnection(modelString), n.chains=3, n.iter=100000, n.burnin=20000, n.thin=100 )
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 713 Initializing model
print(data.jags)
Inference for Bugs model at "7", fit using jags,
3 chains, each with 1e+05 iterations (first 20000 discarded), n.thin = 100
n.sims = 2400 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta1 2.949 0.504 1.971 2.608 2.951 3.284 3.939 1.001 2300
beta2 1.333 0.425 0.484 1.041 1.333 1.618 2.166 1.001 2400
sigma 1.127 0.084 0.977 1.070 1.121 1.181 1.313 1.001 2400
deviance 305.835 3.304 301.533 303.456 305.062 307.486 313.832 1.001 2400
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 5.5 and DIC = 311.3
DIC is an estimate of expected predictive error (lower deviance is better).
#additive model data.inla.a <- inla(y~cx1+cx2, data=data, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE)) #multiplicative model data.inla.m <- inla(y~cx1*cx2, data=data, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE))
We will first of all explore the INLA summaries for the two models, paying particular attention to the KLD, Expected number of effective parameters and number of equivalent replicates estimates.
summary(data.inla.a)
Call:
c("inla(formula = y ~ cx1 + cx2, data = data, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE))")
Time used:
Pre-processing Running inla Post-processing Total
0.0643 0.0534 0.0237 0.1415
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 3.8234 0.1125 3.6022 3.8234 4.0443 3.8234 0
cx1 3.0245 0.4957 2.0500 3.0245 3.9980 3.0245 0
cx2 1.3880 0.4256 0.5513 1.3880 2.2238 1.3880 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for the Gaussian observations 0.7968 0.1133 0.5935 0.7905 1.037 0.7797
Expected number of effective parameters(std dev): 3.001(2e-04)
Number of equivalent replicates : 33.32
Deviance Information Criterion (DIC) ...: 312.74
Effective number of parameters .........: 3.647
Watanabe-Akaike information criterion (WAIC) ...: 312.77
Effective number of parameters .................: 3.546
Marginal log-Likelihood: -173.86
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
summary(data.inla.m)
Call:
c("inla(formula = y ~ cx1 * cx2, data = data, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE))")
Time used:
Pre-processing Running inla Post-processing Total
0.0658 0.0525 0.0239 0.1423
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 3.6712 0.1307 3.4143 3.6712 3.9279 3.6712 0
cx1 2.9288 0.4884 1.9685 2.9288 3.8882 2.9288 0
cx2 1.3446 0.4181 0.5226 1.3446 2.1659 1.3446 0
cx1:cx2 2.6613 1.2224 0.2579 2.6613 5.0621 2.6613 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for the Gaussian observations 0.8273 0.1182 0.6153 0.8208 1.078 0.8094
Expected number of effective parameters(std dev): 3.999(4e-04)
Number of equivalent replicates : 25.00
Deviance Information Criterion (DIC) ...: 309.98
Effective number of parameters .........: 4.648
Watanabe-Akaike information criterion (WAIC) ...: 309.77
Effective number of parameters .................: 4.227
Marginal log-Likelihood: -174.76
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
KLD values for all parameters for both models are 0, and thus the Simplified Laplace Approximation is appropriate. The number of effective parameters for both models is approximately 3. Not surprisingly, the additive model therefore has more equivalent replicates per parameter than the multiplicative model. In either case, there appears to be ample replicates per parameter to ensure reliable parameter estimation.
The multiplicative model has evidence of an interaction (95% credibility interval for the interaction parameter do not overlap zero) and both DIC and WAIC values are lower for the multiplicative model than the additive model. Consequently, the multiplicative model would be considered the better of the two models.
We will examine CPO and PIT from both models.
sum(data.inla.a$cpo$cpo)
[1] 24.45695
-mean(log(data.inla.a$cpo$cpo))
[1] 1.563885
plot(data.inla.a, plot.fixed.effects=FALSE, plot.lincomb=FALSE, plot.random.effects=FALSE, plot.hyperparameters=FALSE, plot.predictor=FALSE, plot.q=FALSE, plot.cpo=TRUE, single=FALSE)
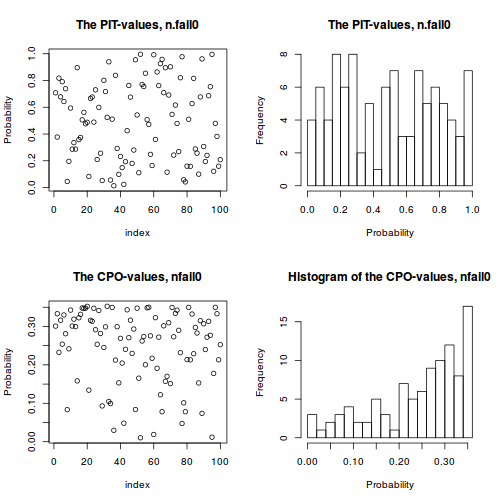
sum(data.inla.m$cpo$cpo)
[1] 24.4262
-mean(log(data.inla.m$cpo$cpo))
[1] 1.54899
plot(data.inla.m, plot.fixed.effects=FALSE, plot.lincomb=FALSE, plot.random.effects=FALSE, plot.hyperparameters=FALSE, plot.predictor=FALSE, plot.q=FALSE, plot.cpo=TRUE, single=FALSE)
Predictions
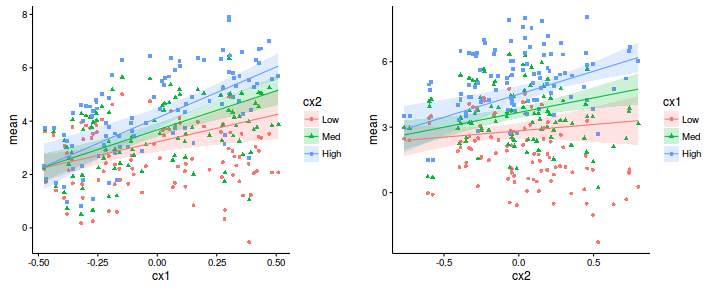
Lets produce partial (conditional) plots for each of the main effects, at a couple of levels of the other covariates (since there was evidence of an interaction). I will do this via the imputation method, however, it could equally be performed via the linear combinations method.
#partial effect of cx1 newdata1 <- expand.grid(cx1=seq(min(data$cx1, na.rm=TRUE), max(data$cx1, na.rm=TRUE), len=100), cx2=c(mean(data$cx2, na.rm=TRUE)-sd(data$cx2, na.rm=TRUE), mean(data$cx2, na.rm=TRUE), mean(data$cx2, na.rm=TRUE)+sd(data$cx2, na.rm=TRUE)), y=NA) #partial residuals of cx1 newdata1r <- expand.grid(cx1=data$cx1, cx2=c(mean(data$cx2, na.rm=TRUE)-sd(data$cx2, na.rm=TRUE), mean(data$cx2, na.rm=TRUE), mean(data$cx2, na.rm=TRUE)+sd(data$cx2, na.rm=TRUE)), y=NA) #partial effect of cx2 newdata2 <- expand.grid(cx2=seq(min(data$cx2, na.rm=TRUE), max(data$cx2, na.rm=TRUE), len=100), cx1=c(mean(data$cx1, na.rm=TRUE)-sd(data$cx1, na.rm=TRUE), mean(data$cx1, na.rm=TRUE), mean(data$cx1, na.rm=TRUE)+sd(data$cx1, na.rm=TRUE)), y=NA) #partial residuals of cx2 newdata2r <- expand.grid(cx2=data$cx2, cx1=c(mean(data$cx1, na.rm=TRUE)-sd(data$cx1, na.rm=TRUE), mean(data$cx1, na.rm=TRUE), mean(data$cx1, na.rm=TRUE)+sd(data$cx1, na.rm=TRUE)), y=NA) newdata <- rbind(data.frame(Pred='cx1', newdata1),data.frame(Pred='cx2', newdata2), data.frame(Pred='cx1r', newdata1r),data.frame(Pred='cx2r', newdata2r)) data.pred <- rbind(data.frame(Pred='Orig',subset(data, select=c(cx1,cx2,y))), newdata) #fit the model data.inla.m1 <- inla(y~cx1*cx2, data=data.pred, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE), control.predictor=list(compute=TRUE)) #extract the predictor values associated with the appended data newdata <- cbind(newdata, data.inla.m1$summary.linear.predictor[(nrow(data)+1):nrow(data.pred),]) newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) newdata1 <- droplevels(subset(newdata, Pred=='cx1')) newdata1$Cov <- factor(newdata1$cx2, labels=c('Low','Med','High')) newdata2 <- droplevels(subset(newdata, Pred=='cx2')) newdata2$Cov <- factor(newdata2$cx1, labels=c('Low','Med','High')) newdata1r <- droplevels(subset(newdata, Pred=='cx1r')) newdata1r$Cov <- factor(newdata1$cx2, labels=c('Low','Med','High')) newdata1r$y <- newdata1r$mean + (newdata1r$mean-data$y) newdata2r <- droplevels(subset(newdata, Pred=='cx2r')) newdata2r$Cov <- factor(newdata2$cx1, labels=c('Low','Med','High')) newdata2r$y <- newdata2r$mean + (newdata2r$mean-data$y) g1<-ggplot(newdata1, aes(y=mean, x=cx1, shape=Cov,fill=Cov, color=Cov)) + geom_point(data=newdata1r, aes(y=y)) + geom_ribbon(aes(ymin=lower, ymax=upper), alpha=0.2, color=NA) + scale_fill_discrete('cx2')+scale_color_discrete('cx2')+scale_shape_discrete('cx2')+ geom_line() + theme_classic() g2<-ggplot(newdata2, aes(y=mean, x=cx2, shape=Cov,fill=Cov, color=Cov)) + geom_point(data=newdata2r, aes(y=y)) + geom_ribbon(aes(ymin=lower, ymax=upper), alpha=0.2, color=NA) + scale_fill_discrete('cx1')+scale_color_discrete('cx1')+scale_shape_discrete('cx1')+ geom_line() + theme_classic() library(gridExtra) grid.arrange(g1,g2, nrow=1)
Single factor ANOVA
Imagine we has designed an experiment in which we had measured the response ($y$) under five different treatments ($x$), each replicated 10 times ($n=10$). As this section is mainly about the generation of artificial data (and not specifically about what to do with the data), understanding the actual details are optional and can be safely skipped. Consequently, I have folded (toggled) this section away.
- the sample size per treatment=10
- the categorical $x$ variable with 5 levels
- the population means associated with the 5 treatment levels are 40, 45, 55, 40, 35 ($\alpha_1=40$, $\alpha_2=45$, $\alpha_3=55$, $\alpha_4=40$, $\alpha_5=30$)
- the data are drawn from normal distributions with a mean of 0 and standard deviation of 3 ($\sigma^2=9$)
set.seed(1) ngroups <- 5 #number of populations nsample <- 10 #number of reps in each pop.means <- c(40, 45, 55, 40, 30) #population mean length sigma <- 3 #residual standard deviation n <- ngroups * nsample #total sample size eps <- rnorm(n, 0, sigma) #residuals x <- gl(ngroups, nsample, n, lab=LETTERS[1:5]) #factor means <- rep(pop.means, rep(nsample, ngroups)) X <- model.matrix(~x-1) #create a design matrix y <- as.numeric(X %*% pop.means+eps) data <- data.frame(y,x) head(data) #print out the first six rows of the data set
y x 1 38.12064 A 2 40.55093 A 3 37.49311 A 4 44.78584 A 5 40.98852 A 6 37.53859 A
$$ y_i = \alpha + \beta X_{i} + \varepsilon_i \hspace{4em}\varepsilon_i\sim{}\mathcal{N}(0, \sigma^2) $$
data.lm <- lm(y~x, data=data) summary(data.lm)
Call:
lm(formula = y ~ x, data = data)
Residuals:
Min 1Q Median 3Q Max
-7.3906 -1.2752 0.3278 1.7931 4.3892
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 40.39661 0.81333 49.668 < 2e-16 ***
xB 5.34993 1.15023 4.651 2.91e-05 ***
xC 14.20237 1.15023 12.347 4.74e-16 ***
xD -0.03442 1.15023 -0.030 0.976
xE -9.99420 1.15023 -8.689 3.50e-11 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 2.572 on 45 degrees of freedom
Multiple R-squared: 0.9129, Adjusted R-squared: 0.9052
F-statistic: 117.9 on 4 and 45 DF, p-value: < 2.2e-16
modelString=" model { #Likelihood for (i in 1:n) { y[i]~dnorm(mean[i],tau) mean[i] <- inprod(beta[],X[i,]) } #Priors for (i in 1:ngroups) { beta[i] ~ dnorm(0, 1.0E-6) } sigma ~ dunif(0, 100) tau <- 1 / (sigma * sigma) #Contrasts Group.means[1] <- beta[1] for (i in 2:ngroups) { Group.means[i] <- beta[i]+beta[1] } #Pairwise effects for (i in 1:ngroups) { for (j in 1:ngroups) { eff[i,j] <- Group.means[i] - Group.means[j] } } #Specific comparisons Comp1 <- Group.means[3] - Group.means[5] Comp2 <- (Group.means[1] + Group.means[2])/2 - (Group.means[3] + Group.means[4] + Group.means[5])/3 } " X <- model.matrix(~x, data) data.list <- with(data, list(y=y, X=X, n=nrow(data), ngroups=ncol(X) ) ) data.jags <- jags(data=data.list, inits=NULL, parameters.to.save=c('beta','sigma', 'eff', 'Comp1', 'Comp2'), model.file=textConnection(modelString), n.chains=3, n.iter=10000, n.burnin=2000, n.thin=100 )
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 406 Initializing model
print(data.jags)
Inference for Bugs model at "6", fit using jags,
3 chains, each with 10000 iterations (first 2000 discarded), n.thin = 100
n.sims = 240 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
Comp1 24.082 1.190 21.973 23.314 24.100 24.837 26.545 1.006 210
Comp2 1.310 0.798 -0.236 0.771 1.382 1.809 2.765 1.001 240
beta[1] 40.440 0.844 38.720 39.847 40.472 40.974 42.135 1.011 140
beta[2] 5.326 1.222 2.887 4.578 5.278 6.102 7.821 1.021 120
beta[3] 14.133 1.224 11.678 13.358 14.106 14.953 16.338 0.999 240
beta[4] -0.124 1.155 -2.139 -0.883 -0.121 0.614 2.279 1.031 120
beta[5] -9.949 1.142 -11.916 -10.737 -9.991 -9.144 -7.772 1.005 240
eff[1,1] 0.000 0.000 0.000 0.000 0.000 0.000 0.000 1.000 1
eff[2,1] 5.326 1.222 2.887 4.578 5.278 6.102 7.821 1.021 120
eff[3,1] 14.133 1.224 11.678 13.358 14.106 14.953 16.338 0.999 240
eff[4,1] -0.124 1.155 -2.139 -0.883 -0.121 0.614 2.279 1.031 120
eff[5,1] -9.949 1.142 -11.916 -10.737 -9.991 -9.144 -7.772 1.005 240
eff[1,2] -5.326 1.222 -7.821 -6.102 -5.278 -4.578 -2.887 1.010 140
eff[2,2] 0.000 0.000 0.000 0.000 0.000 0.000 0.000 1.000 1
eff[3,2] 8.806 1.343 6.244 7.881 8.764 9.745 11.556 1.024 140
eff[4,2] -5.450 1.215 -7.740 -6.381 -5.380 -4.673 -3.025 0.996 240
eff[5,2] -15.276 1.222 -17.554 -15.996 -15.369 -14.446 -12.564 0.996 240
eff[1,3] -14.133 1.224 -16.338 -14.953 -14.106 -13.358 -11.678 0.999 240
eff[2,3] -8.806 1.343 -11.556 -9.745 -8.764 -7.881 -6.244 1.016 140
eff[3,3] 0.000 0.000 0.000 0.000 0.000 0.000 0.000 1.000 1
eff[4,3] -14.257 1.168 -16.241 -15.121 -14.245 -13.474 -11.660 1.018 98
eff[5,3] -24.082 1.190 -26.545 -24.837 -24.100 -23.314 -21.973 1.006 210
eff[1,4] 0.124 1.155 -2.279 -0.614 0.121 0.883 2.139 1.031 120
eff[2,4] 5.450 1.215 3.025 4.673 5.380 6.381 7.740 0.996 240
eff[3,4] 14.257 1.168 11.660 13.474 14.245 15.121 16.241 1.020 94
eff[4,4] 0.000 0.000 0.000 0.000 0.000 0.000 0.000 1.000 1
eff[5,4] -9.825 1.220 -12.114 -10.643 -9.854 -8.901 -7.713 1.001 240
eff[1,5] 9.949 1.142 7.772 9.144 9.991 10.737 11.916 1.005 240
eff[2,5] 15.276 1.222 12.564 14.446 15.369 15.996 17.554 0.996 240
eff[3,5] 24.082 1.190 21.973 23.314 24.100 24.837 26.545 1.006 210
eff[4,5] 9.825 1.220 7.713 8.901 9.854 10.643 12.114 0.999 240
eff[5,5] 0.000 0.000 0.000 0.000 0.000 0.000 0.000 1.000 1
sigma 2.655 0.298 2.153 2.462 2.622 2.823 3.228 0.998 240
deviance 237.918 3.727 232.555 235.288 237.245 239.878 246.813 1.010 170
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 6.9 and DIC = 244.8
DIC is an estimate of expected predictive error (lower deviance is better).
In preparation for fitting this model, we will also consider a number of contrasts (as we did for the Bayesian (JAGS) version of the analysis. Firstly, we will append missing response data in order to generate cell means predictions (for the summary plot) and secondly, we will define linear combinations (contrasts) to provide the pairwise and planned comparisons.
newdata <- data.frame(x=levels(data$x), y=NA) data.pred <- rbind(data, newdata) #Define linear combinations (pairwise comparisons) library(multcomp) tuk.mat <- contrMat(n=table(newdata$x), type="Tukey") Xmat <- model.matrix(~x, data=newdata) pairwise.Xmat <- tuk.mat %*% Xmat pairwise.Xmat
(Intercept) xB xC xD xE B - A 0 1 0 0 0 C - A 0 0 1 0 0 D - A 0 0 0 1 0 E - A 0 0 0 0 1 C - B 0 -1 1 0 0 D - B 0 -1 0 1 0 E - B 0 -1 0 0 1 D - C 0 0 -1 1 0 E - C 0 0 -1 0 1 E - D 0 0 0 -1 1
#Define linear combinations (planned contrasts) comp.Xmat <- rbind(Comp1=c(0,0,1,0,-1), Comp2=c(1/2,1/2,-1/3,-1/3,-1/3)) #make these appropriate for the treatment contrasts applied in the model fit comp.Xmat <-cbind(0,comp.Xmat %*% contr.treatment(5)) rownames(comp.Xmat) <- c("Grp 3 vs 5","Grp 1,2 vs 3,4,5") Xmat <- rbind(pairwise.Xmat, comp.Xmat) Xmat
(Intercept) xB xC xD xE B - A 0 1.0 0.0000000 0.0000000 0.0000000 C - A 0 0.0 1.0000000 0.0000000 0.0000000 D - A 0 0.0 0.0000000 1.0000000 0.0000000 E - A 0 0.0 0.0000000 0.0000000 1.0000000 C - B 0 -1.0 1.0000000 0.0000000 0.0000000 D - B 0 -1.0 0.0000000 1.0000000 0.0000000 E - B 0 -1.0 0.0000000 0.0000000 1.0000000 D - C 0 0.0 -1.0000000 1.0000000 0.0000000 E - C 0 0.0 -1.0000000 0.0000000 1.0000000 E - D 0 0.0 0.0000000 -1.0000000 1.0000000 Grp 3 vs 5 0 0.0 1.0000000 0.0000000 -1.0000000 Grp 1,2 vs 3,4,5 0 0.5 -0.3333333 -0.3333333 -0.3333333
lincomb <- inla.make.lincombs(as.data.frame(Xmat))
Now lets fit the model.
#fit the model data.inla1 <- inla(y~x, lincomb=lincomb, #include the linear combinations data=data.pred, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE), control.inla=list(lincomb.derived.only=TRUE)) #examine the regular summary - not there are no changes to the first fit summary(data.inla1)
Call:
c("inla(formula = y ~ x, data = data.pred, lincomb = lincomb, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE), control.inla = list(lincomb.derived.only = TRUE))" )
Time used:
Pre-processing Running inla Post-processing Total
0.1363 0.0730 0.0552 0.2646
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 40.4027 0.8021 38.8235 40.4026 41.9806 40.4025 0
xB 5.3404 1.1346 3.1059 5.3405 7.5715 5.3407 0
xC 14.1871 1.1346 11.9524 14.1873 16.4180 14.1877 0
xD -0.0405 1.1346 -2.2748 -0.0405 2.1908 -0.0403 0
xE -9.9939 1.1346 -12.2279 -9.9939 -7.7624 -9.9939 0
Linear combinations (derived):
ID mean sd 0.025quant 0.5quant 0.975quant mode kld
lc01 1 5.3404 1.1346 3.1059 5.3405 7.5715 5.3407 0
lc02 2 14.1871 1.1346 11.9524 14.1873 16.4180 14.1877 0
lc03 3 -0.0405 1.1346 -2.2748 -0.0405 2.1908 -0.0403 0
lc04 4 -9.9939 1.1346 -12.2279 -9.9939 -7.7624 -9.9939 0
lc05 5 8.8467 1.1355 6.6106 8.8468 11.0799 8.8470 0
lc06 6 -5.3809 1.1355 -7.6166 -5.3810 -3.1474 -5.3810 0
lc07 7 -15.3342 1.1355 -17.5697 -15.3344 -13.1005 -15.3346 0
lc08 8 -14.2276 1.1355 -16.4632 -14.2278 -11.9939 -14.2280 0
lc09 9 -24.1810 1.1355 -26.4163 -24.1812 -21.9471 -24.1816 0
lc10 10 -9.9534 1.1355 -12.1890 -9.9535 -7.7198 -9.9536 0
lc11 11 24.1810 1.1355 21.9444 24.1812 26.4138 24.1816 0
lc12 12 1.2859 0.7326 -0.1565 1.2859 2.7269 1.2859 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for the Gaussian observations 0.1577 0.0325 0.1018 0.1551 0.229 0.1506
Expected number of effective parameters(std dev): 4.995(9e-04)
Number of equivalent replicates : 10.01
Deviance Information Criterion (DIC) ...: 242.49
Effective number of parameters .........: 5.651
Watanabe-Akaike information criterion (WAIC) ...: 243.15
Effective number of parameters .................: 5.727
Marginal log-Likelihood: -142.21
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
Parameter estimates are entirely consistent with the Frequentist and JAGS outcomes. Lets explore the various contrasts. These are captured in the summary.lincomb.derived element of the INLA list. To make this a little more informative (so we can see what each contrast represents, I will apply the rownames from the Xmat.
rownames(data.inla1$summary.lincomb.derived) <- rownames(Xmat) data.inla1$summary.lincomb.derived
ID mean sd 0.025quant 0.5quant 0.975quant mode kld B - A 1 5.34035821 1.1345555 3.1059307 5.34045527 7.571501 5.34073924 0 C - A 2 14.18709661 1.1345572 11.9524362 14.18727158 16.418038 14.18770537 0 D - A 3 -0.04051494 1.1345548 -2.2748016 -0.04046525 2.190752 -0.04027242 0 E - A 4 -9.99387467 1.1345544 -12.2279022 -9.99391263 -7.762378 -9.99388837 0 C - B 5 8.84673840 1.1354933 6.6105973 8.84678312 11.079883 8.84696648 0 D - B 6 -5.38087314 1.1354931 -7.6166440 -5.38095391 -3.147399 -5.38101187 0 E - B 7 -15.33423287 1.1354941 -17.5697471 -15.33440142 -13.100526 -15.33462821 0 D - C 8 -14.22761155 1.1354940 -16.4631542 -14.22777033 -11.993931 -14.22797835 0 E - C 9 -24.18097128 1.1354959 -26.4162589 -24.18121785 -21.947056 -24.18159469 0 E - D 10 -9.95335973 1.1354934 -12.1890125 -9.95348082 -7.719779 -9.95361634 0 Grp 3 vs 5 11 24.18097128 1.1354959 21.9444267 24.18115124 26.413765 24.18159469 0 Grp 1,2 vs 3,4,5 12 1.28594339 0.7325942 -0.1565176 1.28589156 2.726922 1.28585466 0
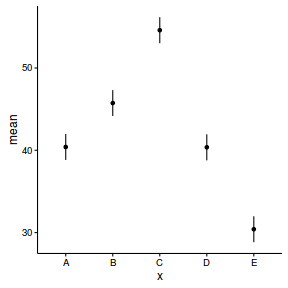
Finally, it is time to extract the predicted values from the linear predictor and plot the cell means.
#summary figure newdata <- cbind(newdata, data.inla1$summary.linear.predictor[(nrow(data)+1):nrow(data.pred),]) newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) ggplot(newdata, aes(y=mean, x=x)) + geom_linerange(aes(ymin=lower, ymax=upper))+ geom_point() + theme_classic()
Factorial ANOVA
Imagine we has designed an experiment in which we had measured the response ($y$) under a combination of two different potential influences (Factor A: levels $a1$ and $a2$; and Factor B: levels $b1$, $b2$ and $b3$), each combination replicated 10 times ($n=10$). As this section is mainly about the generation of artificial data (and not specifically about what to do with the data), understanding the actual details are optional and can be safely skipped. Consequently, I have folded (toggled) this section away.
- the sample size per treatment=10
- factor A with 2 levels
- factor B with 3 levels
- the 6 effects parameters are 40, 15, 5, 0, -15,10 ($\mu_{a1b1}=40$, $\mu_{a2b1}=40+15=55$, $\mu_{a1b2}=40+5=45$, $\mu_{a1b3}=40+0=40$, $\mu_{a2b2}=40+15+5-15=45$ and $\mu_{a2b3}=40+15+0+10=65$)
- the data are drawn from normal distributions with a mean of 0 and standard deviation of 3 ($\sigma^2=9$)
set.seed(1) nA <- 2 #number of levels of A nB <- 3 #number of levels of B nsample <- 10 #number of reps in each A <- gl(nA,1,nA, lab=paste("a",1:nA,sep="")) B <- gl(nB,1,nB, lab=paste("b",1:nB,sep="")) data <-expand.grid(A=A,B=B, n=1:nsample) X <- model.matrix(~A*B, data=data) eff <- c(40,15,5,0,-15,10) sigma <- 3 #residual standard deviation n <- nrow(data) eps <- rnorm(n, 0, sigma) #residuals data$y <- as.numeric(X %*% eff+eps) head(data) #print out the first six rows of the data set
A B n y 1 a1 b1 1 38.12064 2 a2 b1 1 55.55093 3 a1 b2 1 42.49311 4 a2 b2 1 49.78584 5 a1 b3 1 40.98852 6 a2 b3 1 62.53859
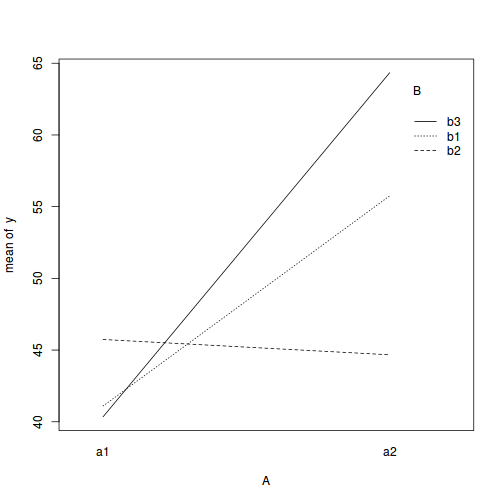
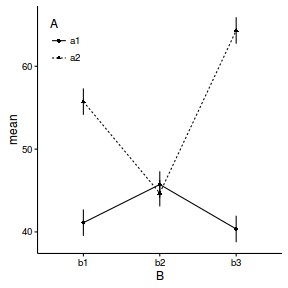
with(data,interaction.plot(A,B,y))
## ALTERNATIVELY, we could supply the population means ## and get the effect parameters from these. ## To correspond to the model matrix, enter the population ## means in the order of: ## a1b1, a2b1, a1b1, a2b2,a1b3,a2b3 pop.means <- as.matrix(c(40,55,45,45,40,65),byrow=F) ## Generate a minimum model matrix for the effects XX <-model.matrix(~A*B, expand.grid(A=factor(1:2),B=factor(1:3))) ## Use the solve() function to solve what are effectively simultaneous equations (eff<-as.vector(solve(XX,pop.means)))
[1] 40 15 5 0 -15 10
data$y <- as.numeric(X %*% eff+eps)
$$ y_i = \alpha + \beta X_{i} + \varepsilon_i \hspace{4em}\varepsilon_i\sim{}\mathcal{N}(0, \sigma^2) $$
data.lm <- lm(y~A*B, data=data) summary(data.lm)
Call:
lm(formula = y ~ A * B, data = data)
Residuals:
Min 1Q Median 3Q Max
-7.3944 -1.5753 0.2281 1.5575 5.1909
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 41.0988 0.8218 50.010 < 2e-16 ***
Aa2 14.6515 1.1622 12.606 < 2e-16 ***
Bb2 4.6386 1.1622 3.991 0.0002 ***
Bb3 -0.7522 1.1622 -0.647 0.5202
Aa2:Bb2 -15.7183 1.6436 -9.563 3.24e-13 ***
Aa2:Bb3 9.3352 1.6436 5.680 5.54e-07 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 2.599 on 54 degrees of freedom
Multiple R-squared: 0.9245, Adjusted R-squared: 0.9175
F-statistic: 132.3 on 5 and 54 DF, p-value: < 2.2e-16
modelString=" model { #Likelihood for (i in 1:n) { y[i]~dnorm(mean[i],tau) mean[i] <- inprod(beta[],X[i,]) } #Priors and derivatives for (i in 1:p) { beta[i] ~ dnorm(0, 1.0E-6) #prior } sigma ~ dunif(0, 100) tau <- 1 / (sigma * sigma) } " X <- model.matrix(~A*B, data) data.list <- with(data, list(y=y, X=X, n=nrow(data), p=ncol(X) ) ) data.jags <- jags(data=data.list, inits=NULL, parameters.to.save=c('beta','sigma'), model.file=textConnection(modelString), n.chains=3, n.iter=10000, n.burnin=2000, n.thin=100 )
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 502 Initializing model
print(data.jags)
Inference for Bugs model at "8", fit using jags,
3 chains, each with 10000 iterations (first 2000 discarded), n.thin = 100
n.sims = 240 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta[1] 40.999 0.894 39.444 40.348 40.972 41.622 42.780 1.005 240
beta[2] 14.849 1.136 12.625 14.026 14.859 15.554 16.912 0.997 240
beta[3] 4.793 1.204 2.600 4.029 4.806 5.659 6.884 1.012 240
beta[4] -0.646 1.214 -3.104 -1.375 -0.585 0.182 1.544 0.996 240
beta[5] -16.021 1.739 -19.540 -16.976 -15.918 -14.945 -12.609 0.996 240
beta[6] 9.216 1.565 6.271 8.162 9.269 10.179 12.699 1.002 240
sigma 2.665 0.281 2.238 2.446 2.650 2.837 3.309 1.000 240
deviance 286.159 3.935 280.494 283.270 285.659 288.017 293.962 1.017 210
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 7.7 and DIC = 293.9
DIC is an estimate of expected predictive error (lower deviance is better).
newdata <- expand.grid(A=levels(data$A), B=levels(data$B), y=NA) data.pred <- rbind(subset(data, select=c(A,B,y)), newdata)
In addition to fitting the model in INLA, it might be useful to also derive the marginal effects of one of the factors (A) at each level of the other factor (B).
#Define linear combinations (planned contrasts) Xmat <- model.matrix(~A*B, data=newdata) cbind(newdata, Xmat)
A B y (Intercept) Aa2 Bb2 Bb3 Aa2:Bb2 Aa2:Bb3 1 a1 b1 NA 1 0 0 0 0 0 2 a2 b1 NA 1 1 0 0 0 0 3 a1 b2 NA 1 0 1 0 0 0 4 a2 b2 NA 1 1 1 0 1 0 5 a1 b3 NA 1 0 0 1 0 0 6 a2 b3 NA 1 1 0 1 0 1
library(plyr) Xmat <- daply(cbind(newdata, Xmat), ~B, function(x) { x[2,] - x[1,] }) Xmat
B A B y (Intercept) Aa2 Bb2 Bb3 Aa2:Bb2 Aa2:Bb3 b1 NA NA NA 0 1 0 0 0 0 b2 NA NA NA 0 1 0 0 1 0 b3 NA NA NA 0 1 0 0 0 1
lincomb <- inla.make.lincombs(as.data.frame(Xmat[,-1:-3]))
Now lets fit the model.
#fit the model data.inla <- inla(y~A*B, data=data.pred, lincomb=lincomb, control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE), control.inla=list(lincomb.derived.only=TRUE)) #examine the regular summary - not there are no changes to the first fit summary(data.inla)
Call:
c("inla(formula = y ~ A * B, data = data.pred, lincomb = lincomb, ", " control.compute = list(dic = TRUE, cpo = TRUE, waic = TRUE), ", " control.inla = list(lincomb.derived.only = TRUE))")
Time used:
Pre-processing Running inla Post-processing Total
0.0820 0.0597 0.0309 0.1727
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 41.1152 0.8119 39.5179 41.1150 42.7118 41.1147 0
Aa2 14.6213 1.1474 12.3621 14.6216 16.8761 14.6223 0
Bb2 4.6088 1.1479 2.3485 4.6091 6.8648 4.6098 0
Bb3 -0.7619 1.1479 -3.0216 -0.7619 1.4946 -0.7616 0
Aa2:Bb2 -15.6643 1.6225 -18.8558 -15.6650 -12.4729 -15.6661 0
Aa2:Bb3 9.3525 1.6225 6.1598 9.3523 12.5428 9.3519 0
Linear combinations (derived):
ID mean sd 0.025quant 0.5quant 0.975quant mode kld
lc1 1 14.6213 1.1474 12.3621 14.6216 16.8761 14.6223 0
lc2 2 -1.0430 1.1491 -3.3039 -1.0434 1.2168 -1.0438 0
lc3 3 23.9738 1.1491 21.7117 23.9739 26.2326 23.9742 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for the Gaussian observations 0.1534 0.029 0.103 0.1513 0.2164 0.1476
Expected number of effective parameters(std dev): 5.991(0.0014)
Number of equivalent replicates : 10.02
Deviance Information Criterion (DIC) ...: 292.00
Effective number of parameters .........: 6.649
Watanabe-Akaike information criterion (WAIC) ...: 292.64
Effective number of parameters .................: 6.634
Marginal log-Likelihood: -169.80
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
Finally, it is time to extract the predicted values from the linear predictor and plot the cell means.
#summary figure newdata <- cbind(newdata, data.inla$summary.linear.predictor[(nrow(data)+1):nrow(data.pred),]) newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) head(newdata)
A B y mean sd lower 0.5quant upper mode kld predictor.61 a1 b1 NA 41.11522 0.8118338 39.52008 41.11503 42.71099 41.11468 1.674684e-09 predictor.62 a2 b1 NA 55.73648 0.8123780 54.13916 55.73664 57.33253 55.73696 1.885519e-09 predictor.63 a1 b2 NA 45.72401 0.8126511 44.12616 45.72417 47.32061 45.72447 1.878400e-09 predictor.64 a2 b2 NA 44.68097 0.8129257 43.08342 44.68085 46.27874 44.68063 1.702529e-09 predictor.65 a1 b3 NA 40.35329 0.8126503 38.75616 40.35321 41.95042 40.35307 1.732000e-09 predictor.66 a2 b3 NA 64.32707 0.8129237 62.72894 64.32714 65.92440 64.32729 1.822437e-09
ggplot(newdata, aes(y=mean, x=B, shape=A)) + geom_linerange(aes(ymin=lower, ymax=upper))+ geom_point() + geom_line(aes(x=as.numeric(B), linetype=A)) + theme_classic()+ theme(legend.position=c(0,1),legend.justification=c(0,1))
Logistic regression (binary response)
Lets say we wanted to model the presence/absence of an item ($y$) against a continuous predictor ($x$) As this section is mainly about the generation of artificial data (and not specifically about what to do with the data), understanding the actual details are optional and can be safely skipped. Consequently, I have folded (toggled) this section away.
- the sample size = 20
- the continuous $x$ variable is a random uniform spread of measurements between 1 and 20
- the rate of change in log odds ratio per unit change in x (slope) = 0.5. The magnitude of the slope is an indicator of how abruptly the log odds ratio changes. A very abrupt change would occur if there was very little variability in the trend. That is, a large slope would be indicative of a threshold effect. A lower slope indicates a gradual change in odds ratio and thus a less obvious and precise switch between the likelihood of 1 vs 0.
- the inflection point (point where the slope of the line is greatest) = 10. The inflection point indicates the value of the predictor ($x$) where the log Odds are 50% (switching between 1 being more likely and 0 being more likely). This is also known as the LD50.
- the intercept (which has no real interpretation) is equal to the negative of the slope multiplied by the inflection point
- to generate the values of $y$ expected at each $x$ value, we evaluate the linear predictor. These expected values are then transformed into a scale mapped by (0,1) by using the inverse logit function $\frac{e^{linear~predictor}}{1+e^{linear~predictor}}$
- finally, we generate $y$ values by using the expected $y$ values as probabilities when drawing random numbers from a binomial distribution. This step adds random noise to the expected $y$ values and returns only 1's and 0's.
set.seed(8) #The number of samples n.x <- 20 #Create x values that at uniformly distributed throughout the rate of 1 to 20 x <- sort(runif(n = n.x, min = 1, max =20)) #The slope is the rate of change in log odds ratio for each unit change in x # the smaller the slope, the slower the change (more variability in data too) slope=0.5 #Inflection point is where the slope of the line is greatest #this is also the LD50 point inflect <- 10 #Intercept (no interpretation) intercept <- -1*(slope*inflect) #The linear predictor linpred <- intercept+slope*x #Predicted y values y.pred <- exp(linpred)/(1+exp(linpred)) #Add some noise and make binomial n.y <-rbinom(n=n.x,20,p=0.9) y<- rbinom(n = n.x,size=1, prob = y.pred) dat <- data.frame(y,x)
$$ log\left(\frac{\pi}{1-\pi}\right)=\beta_0+\beta_1x_1+\epsilon_i, \hspace{1cm}\epsilon\sim Binom(\lambda) $$
dat.glm <- glm(y~x, data=dat, family="binomial") summary(dat.glm)
Call:
glm(formula = y ~ x, family = "binomial", data = dat)
Deviance Residuals:
Min 1Q Median 3Q Max
-1.97157 -0.33665 -0.08191 0.30035 1.59628
Coefficients:
Estimate Std. Error z value Pr(>|z|)
(Intercept) -6.9899 3.1599 -2.212 0.0270 *
x 0.6559 0.2936 2.234 0.0255 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
(Dispersion parameter for binomial family taken to be 1)
Null deviance: 27.526 on 19 degrees of freedom
Residual deviance: 11.651 on 18 degrees of freedom
AIC: 15.651
Number of Fisher Scoring iterations: 6
modelString=" model{ for (i in 1:N) { y[i] ~ dbern(p[i]) logit(p[i]) <- max(-20,min(20,beta0+beta1*x[i])) } beta0 ~ dnorm(0,1.0E-06) beta1 ~ dnorm(0,1.0E-06) } " dat.list <- with(dat, list(y=y, x=x, N=nrow(dat))) dat.jags <- jags(data=dat.list,model.file=textConnection(modelString), param=c('beta0','beta1'), n.chains=3, n.iter=20000, n.burnin=10000, n.thin=100)
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 147 Initializing model
print(dat.jags)
Inference for Bugs model at "9", fit using jags,
3 chains, each with 20000 iterations (first 10000 discarded), n.thin = 100
n.sims = 300 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta0 -9.497 3.970 -18.493 -12.068 -8.922 -6.449 -3.740 1.004 300
beta1 0.898 0.370 0.353 0.612 0.844 1.123 1.772 1.003 300
deviance 13.872 2.108 11.683 12.301 13.291 14.811 19.290 1.018 200
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 2.2 and DIC = 16.1
DIC is an estimate of expected predictive error (lower deviance is better).
library(rstan) modelString=" data { int<lower=1> n; int<lower=1> nX; int y[n]; matrix [n,nX] X; } parameters { vector [nX] beta; } transformed parameters { vector [n] mu; mu<-X*beta; } model { beta ~ normal(0,1000); y ~ binomial_logit(1, mu); } " Xmat <- model.matrix(~x,data=dat) dat.list <- with(dat, list(y = y, X = Xmat, nX=ncol(Xmat),n = nrow(dat))) library(rstan) dat.rstan <- stan(data=dat.list, model_code=modelString, chains=3, iter=1000, warmup=500, thin=2, save_dso=TRUE )
SAMPLING FOR MODEL '8378cae68bcb88c343bcb3d714aeb387' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.060304 seconds (Warm-up) # 0.053233 seconds (Sampling) # 0.113537 seconds (Total) # SAMPLING FOR MODEL '8378cae68bcb88c343bcb3d714aeb387' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.078958 seconds (Warm-up) # 0.046298 seconds (Sampling) # 0.125256 seconds (Total) # SAMPLING FOR MODEL '8378cae68bcb88c343bcb3d714aeb387' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.063185 seconds (Warm-up) # 0.053685 seconds (Sampling) # 0.11687 seconds (Total) #
print(dat.rstan, pars=c('beta'))
Inference for Stan model: 8378cae68bcb88c343bcb3d714aeb387.
3 chains, each with iter=1000; warmup=500; thin=2;
post-warmup draws per chain=250, total post-warmup draws=750.
mean se_mean sd 2.5% 25% 50% 75% 97.5% n_eff Rhat
beta[1] -9.56 0.28 3.86 -18.39 -11.73 -9.04 -6.74 -3.78 189 1
beta[2] 0.90 0.03 0.36 0.36 0.64 0.85 1.09 1.71 193 1
Samples were drawn using NUTS(diag_e) at Mon Feb 22 12:02:04 2016.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).
library(brms) dat.brm <- brm(y~x, data=dat, family='binomial', prior=c(set_prior('normal(0,1000)', class='b')), n.chains=3, n.iter=1000, warmup=500, n.thin=2 )
SAMPLING FOR MODEL 'binomial(logit) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.061118 seconds (Warm-up) # 0.038603 seconds (Sampling) # 0.099721 seconds (Total) # SAMPLING FOR MODEL 'binomial(logit) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.070109 seconds (Warm-up) # 0.048011 seconds (Sampling) # 0.11812 seconds (Total) # SAMPLING FOR MODEL 'binomial(logit) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.130434 seconds (Warm-up) # 0.058031 seconds (Sampling) # 0.188465 seconds (Total) #
summary(dat.brm)
Family: binomial (logit)
Formula: y ~ x
Data: dat (Number of observations: 20)
Samples: 3 chains, each with n.iter = 1000; n.warmup = 500; n.thin = 2;
total post-warmup samples = 750
WAIC: 17.16
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
Intercept -9.43 4.09 -18.32 -2.80 219 1
x 0.89 0.38 0.29 1.68 215 1
Samples were drawn using NUTS(diag_e). For each parameter, Eff.Sample is a
crude measure of effective sample size, and Rhat is the potential scale
reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
}
data {
int<lower=1> N; # number of observations
int Y[N]; # response variable
int<lower=1> K; # number of fixed effects
matrix[N, K] X; # FE design matrix
int trials; # number of trials
}
transformed data {
}
parameters {
real b_Intercept; # fixed effects Intercept
vector[K] b; # fixed effects
}
transformed parameters {
vector[N] eta; # linear predictor
# compute linear predictor
eta <- X * b + b_Intercept;
}
model {
# prior specifications
b_Intercept ~ normal(0,1000);
b ~ normal(0,1000);
# likelihood contribution
Y ~ binomial_logit(trials, eta);
}
generated quantities {
}
fitted <- subset(dat, select=c(x,y)) fitted$y <- NA newdata <- expand.grid(x=seq(min(dat$x), max(dat$x), len=100), y=NA) dat.pred <- rbind(dat,fitted,newdata)
Now lets fit the model.
#fit the model dat.inla <- inla(y~x, family='binomial', data=dat.pred, control.family=list(link='logit'), control.predictor=list(link=1, compute=TRUE), control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE)) #examine the regular summary summary(dat.inla)
Call:
c("inla(formula = y ~ x, family = \"binomial\", data = dat.pred, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE), control.predictor = list(link = 1, ", " compute = TRUE), control.family = list(link = \"logit\"))" )
Time used:
Pre-processing Running inla Post-processing Total
0.0679 0.0406 0.0192 0.1277
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) -7.5996 3.1591 -14.6917 -7.2414 -2.3638 -6.4312 0
x 0.7134 0.2935 0.2284 0.6797 1.3727 0.6036 0
The model has no random effects
The model has no hyperparameters
Expected number of effective parameters(std dev): 2.00(0.00)
Number of equivalent replicates : 10.00
Deviance Information Criterion (DIC) ...: 15.37
Effective number of parameters .........: 1.847
Watanabe-Akaike information criterion (WAIC) ...: 15.74
Effective number of parameters .................: 1.875
Marginal log-Likelihood: -9.883
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
newdata <- cbind(newdata, dat.inla$summary.fitted.values[(nrow(dat)+nrow(fitted)+1):nrow(dat.pred),]) head(newdata)
x y mean sd 0.025quant 0.5quant 0.975quant mode fitted.predictor.041 1.024733 NA 0.01285490 0.03964898 1.677056e-06 0.001438164 0.1066886 2.284295e-10 fitted.predictor.042 1.203403 NA 0.01350352 0.04040270 2.134741e-06 0.001623817 0.1109843 5.081955e-10 fitted.predictor.043 1.382074 NA 0.01419668 0.04119379 2.716943e-06 0.001833402 0.1154430 1.082068e-09 fitted.predictor.044 1.560745 NA 0.01493801 0.04202428 3.457403e-06 0.002069996 0.1200704 2.214625e-09 fitted.predictor.045 1.739415 NA 0.01573143 0.04289634 4.398962e-06 0.002337068 0.1248719 4.387127e-09 fitted.predictor.046 1.918086 NA 0.01658128 0.04381226 5.595989e-06 0.002638525 0.1298534 8.434306e-09
newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) #fitted <- cbind(fitted, # dat.inla$summary.fitted.values[(nrow(dat)+1):(nrow(dat)+nrow(fitted)),]) #fitted <- reshape:::rename(fitted, c("0.025quant"="lower", "0.975quant"="upper")) #fitted$partial.obs=fitted$mean + (fitted$mean - dat$y) #ndata <- data.splt #ndata$fit <- fitted$mean #ndata$pred <- pred$mean #ndata$Res <- (ndata$fit - data.splt$y) #ndata$Pobs <- ndata$pred + ndata$Res #head(ndata) library(grid) ggplot(newdata, aes(y=mean, x=x)) + geom_blank()+ geom_point(data=dat, aes(y=y, x=x)) + geom_ribbon(aes(ymin=lower, ymax=upper), fill='blue', alpha=0.2) + geom_line() + theme_classic()+ theme(legend.key.width=unit(2,'lines'), legend.position=c(0,1), legend.justification=c(0,1))
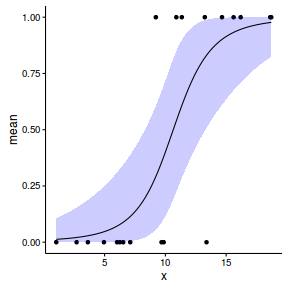
Grouped binomial regression (proportional counts)
Recall that the binomial distribution represents the density (probability) of all possible successes out of a total of N trials. Hence the binomial distribution is also a suitable error distribution for analysing grouped binary data. An example of group data might be the number of seeds that germinate out of a total of 10 seeds, or the number of female kangaroos with joeys out of a total of 120 female kangaroos.
For this demonstration, we will model the number of successes against a uniformly distributed predictor (x). The number of trials in each group (level of the predictor) will vary slightly (yet randomly) so as to mimic complications that inevitable occur in real experiments.
- the number of levels of $x$ = 10
- the continuous $x$ variable is a random uniform spread of measurements between 10 and 20
- the rate of change in log odds ratio per unit change in x (slope) = -0.25. The magnitude of the slope is an indicator of how abruptly the log odds ratio changes. A very abrupt change would occur if there was very little variability in the trend. That is, a large slope would be indicative of a threshold effect. A lower slope indicates a gradual change in odds ratio and thus a less obvious and precise switch between the likelihood of 1 vs 0.
- the inflection point (point where the slope of the line is greatest) = 10. The inflection point indicates the value of the predictor ($x$) where the log Odds are 50% (switching between 1 being more likely and 0 being more likely). This is also known as the LD50.
- the intercept (which has no real interpretation) is equal to the negative of the slope multiplied by the inflection point
- generate the values of $y$ expected at each $x$ value, we evaluate the linear predictor.
- generate random numbers of trials per group by drawing integers out of a binomial distribution with a total size of 20 and probability of 0.9. Hence the maximum number of trials per group will be 20, yet some will be less than that.
- the number of success per group are drawn from a binomial distribution with a total size equal to the trial sizes generated in the previous step and probabilities equal to the expected $y$ values
- finally, the number of failures per group are calculated as the difference between the total trial size and number of successes per group.
set.seed(8) #The number of levels of x n.x <- 10 #Create x values that at uniformly distributed throughout the rate of 10 to 20 x <- sort(runif(n = n.x, min = 10, max =20)) #The slope is the rate of change in log odds ratio for each unit change in x # the smaller the slope, the slower the change (more variability in data too) slope=-.25 #Inflection point is where the slope of the line is greatest #this is also the LD50 point inflect <- 15 #Intercept (no interpretation) intercept <- -1*(slope*inflect) #The linear predictor linpred <- intercept+slope*x #Predicted y values y.pred <- exp(linpred)/(1+exp(linpred)) #Add some noise and make binary (0's and 1's) n.trial <- rbinom(n=n.x,20, prob=0.9) success <- rbinom(n = n.x, size = n.trial,prob = y.pred) failure <- n.trial - success dat <- data.frame(success,failure,x)
$$ log\left(\frac{\pi}{1-\pi}\right)=\beta_0+\beta_1x_1+\epsilon_i, \hspace{1cm}\epsilon\sim Binom(\lambda) $$
dat<-within(dat, total<-success+failure) dat.glm <- glm(cbind(success,failure)~x, data=dat, family="binomial", weight=total) summary(dat.glm)
Call:
glm(formula = cbind(success, failure) ~ x, family = "binomial",
data = dat, weights = total)
Deviance Residuals:
Min 1Q Median 3Q Max
-6.3073 -1.2530 0.5005 2.1157 5.8746
Coefficients:
Estimate Std. Error z value Pr(>|z|)
(Intercept) 4.0472 0.2525 16.03 <2e-16 ***
x -0.2591 0.0158 -16.40 <2e-16 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
(Dispersion parameter for binomial family taken to be 1)
Null deviance: 394.60 on 9 degrees of freedom
Residual deviance: 105.33 on 8 degrees of freedom
AIC: 716.15
Number of Fisher Scoring iterations: 3
dat.list <- with(dat, list(success=success, total=success+failure, x=x, N=nrow(dat))) modelString=" model{ for (i in 1:N) { success[i] ~ dbin(p[i],total[i]) logit(p[i]) <- max(-20,min(20,beta0+beta1*x[i])) } beta0 ~ dnorm(0,1.0E-06) beta1 ~ dnorm(0,1.0E-06) } " writeLines(modelString, con='../downloads/BUGSscripts/tut11.4bS2.bug') library(R2jags) dat.logit.jags <- jags(data=dat.list,model.file='../downloads/BUGSscripts/tut11.4bS2.bug', param=c('beta0','beta1'), n.chains=3, n.iter=200000, n.burnin=100000, n.thin=100)
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 87 Initializing model
print(dat.logit.jags)
Inference for Bugs model at "../downloads/BUGSscripts/tut11.4bS2.bug", fit using jags,
3 chains, each with 2e+05 iterations (first 1e+05 discarded), n.thin = 100
n.sims = 3000 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta0 4.008 1.086 1.892 3.305 3.972 4.753 6.148 1.002 1300
beta1 -0.256 0.068 -0.387 -0.302 -0.255 -0.210 -0.125 1.002 1300
deviance 40.637 1.984 38.680 39.218 40.011 41.478 45.896 1.001 3000
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 2.0 and DIC = 42.6
DIC is an estimate of expected predictive error (lower deviance is better).
library(rstan) modelString=" data { int<lower=1> n; int<lower=1> nX; int y[n]; int nTrials[n]; matrix [n,nX] X; } parameters { vector [nX] beta; } transformed parameters { vector [n] mu; mu<-X*beta; } model { beta ~ normal(0,1000); y ~ binomial_logit(nTrials, mu); } " Xmat <- model.matrix(~x,data=dat) dat.list <- with(dat, list(y = success, nTrials=success+failure, X = Xmat, nX=ncol(Xmat),n = nrow(dat))) library(rstan) dat.rstan <- stan(data=dat.list, model_code=modelString, chains=3, iter=1000, warmup=500, thin=2, save_dso=TRUE )
SAMPLING FOR MODEL '6c0358c6c330110e714238536a9a5894' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.060866 seconds (Warm-up) # 0.032433 seconds (Sampling) # 0.093299 seconds (Total) # SAMPLING FOR MODEL '6c0358c6c330110e714238536a9a5894' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.055367 seconds (Warm-up) # 0.038589 seconds (Sampling) # 0.093956 seconds (Total) # SAMPLING FOR MODEL '6c0358c6c330110e714238536a9a5894' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.126566 seconds (Warm-up) # 0.072445 seconds (Sampling) # 0.199011 seconds (Total) #
print(dat.rstan, pars=c('beta'))
Inference for Stan model: 6c0358c6c330110e714238536a9a5894.
3 chains, each with iter=1000; warmup=500; thin=2;
post-warmup draws per chain=250, total post-warmup draws=750.
mean se_mean sd 2.5% 25% 50% 75% 97.5% n_eff Rhat
beta[1] 4.16 0.07 1.08 2.07 3.41 4.19 4.84 6.34 219 1.00
beta[2] -0.26 0.00 0.07 -0.39 -0.31 -0.27 -0.22 -0.13 217 1.01
Samples were drawn using NUTS(diag_e) at Mon Feb 22 12:12:59 2016.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).
library(brms) dat<-within(dat, total<-success+failure) dat.brm <- brm(success|trials(total)~x, data=dat, family='binomial', prior=c(set_prior('normal(0,1000)', class='b')), n.chains=3, n.iter=1000, warmup=500, n.thin=2 )
SAMPLING FOR MODEL 'binomial(logit) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.061524 seconds (Warm-up) # 0.040658 seconds (Sampling) # 0.102182 seconds (Total) # SAMPLING FOR MODEL 'binomial(logit) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.073432 seconds (Warm-up) # 0.039209 seconds (Sampling) # 0.112641 seconds (Total) # SAMPLING FOR MODEL 'binomial(logit) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.061522 seconds (Warm-up) # 0.036074 seconds (Sampling) # 0.097596 seconds (Total) #
summary(dat.brm)
Family: binomial (logit)
Formula: success | trials(total) ~ x
Data: dat (Number of observations: 10)
Samples: 3 chains, each with n.iter = 1000; n.warmup = 500; n.thin = 2;
total post-warmup samples = 750
WAIC: 41.57
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
Intercept 4.10 1.00 2.24 6.00 200 1
x -0.26 0.06 -0.38 -0.14 200 1
Samples were drawn using NUTS(diag_e). For each parameter, Eff.Sample is a
crude measure of effective sample size, and Rhat is the potential scale
reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
}
data {
int<lower=1> N; # number of observations
int Y[N]; # response variable
int<lower=1> K; # number of fixed effects
matrix[N, K] X; # FE design matrix
int trials[N]; # number of trials
}
transformed data {
}
parameters {
real b_Intercept; # fixed effects Intercept
vector[K] b; # fixed effects
}
transformed parameters {
vector[N] eta; # linear predictor
# compute linear predictor
eta <- X * b + b_Intercept;
}
model {
# prior specifications
b_Intercept ~ normal(0,1000);
b ~ normal(0,1000);
# likelihood contribution
Y ~ binomial_logit(trials, eta);
}
generated quantities {
}
fitted <- subset(dat, select=c(x,success,failure,total)) fitted$success <- NA newdata <- expand.grid(success=NA, failure=NA,x=seq(min(dat$x), max(dat$x), len=100), total=NA) dat.pred <- rbind(dat,fitted,newdata)
Now lets fit the model.
#fit the model dat.inla <- inla(success~x, family='binomial',Ntrials=total, data=dat.pred, control.family=list(link='logit'), control.predictor=list(link=1, compute=TRUE), control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE)) #examine the regular summary summary(dat.inla)
Call:
c("inla(formula = success ~ x, family = \"binomial\", data = dat.pred, ", " Ntrials = total, control.compute = list(dic = TRUE, cpo = TRUE, ", " waic = TRUE), control.predictor = list(link = 1, compute = TRUE), ", " control.family = list(link = \"logit\"))")
Time used:
Pre-processing Running inla Post-processing Total
0.0825 0.0438 0.0288 0.1551
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 4.0391 1.088 1.9497 4.0230 6.2217 3.9907 0
x -0.2577 0.068 -0.3940 -0.2567 -0.1266 -0.2548 0
The model has no random effects
The model has no hyperparameters
Expected number of effective parameters(std dev): 2.001(0.00)
Number of equivalent replicates : 4.999
Deviance Information Criterion (DIC) ...: 42.59
Effective number of parameters .........: 1.987
Watanabe-Akaike information criterion (WAIC) ...: 41.93
Effective number of parameters .................: 1.15
Marginal log-Likelihood: -26.41
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
newdata <- cbind(newdata, dat.inla$summary.fitted.values[(nrow(dat)+nrow(fitted)+1):nrow(dat.pred),]) head(newdata)
success failure x total mean sd 0.025quant 0.5quant 0.975quant
fitted.predictor.021 NA NA 12.07823 NA 0.7125421 0.05985063 0.5878929 0.7153579 0.8212576
fitted.predictor.022 NA NA 12.15141 NA 0.7088373 0.05946393 0.5852983 0.7115181 0.8171994
fitted.predictor.023 NA NA 12.22459 NA 0.7050996 0.05906512 0.5826879 0.7076480 0.8130769
fitted.predictor.024 NA NA 12.29776 NA 0.7013292 0.05865439 0.5800613 0.7037477 0.8088906
fitted.predictor.025 NA NA 12.37094 NA 0.6975263 0.05823199 0.5774180 0.6998179 0.8046408
fitted.predictor.026 NA NA 12.44412 NA 0.6936912 0.05779817 0.5747575 0.6958587 0.8003281
mode
fitted.predictor.021 0.7212332
fitted.predictor.022 0.7171021
fitted.predictor.023 0.7129469
fitted.predictor.024 0.7087681
fitted.predictor.025 0.7045663
fitted.predictor.026 0.7003420
newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) #fitted <- cbind(fitted, # dat.inla$summary.fitted.values[(nrow(dat)+1):(nrow(dat)+nrow(fitted)),]) #fitted <- reshape:::rename(fitted, c("0.025quant"="lower", "0.975quant"="upper")) #fitted$partial.obs=fitted$mean + (fitted$mean - dat$y) library(grid) ggplot(newdata, aes(y=mean, x=x)) + geom_blank()+ geom_point(data=dat, aes(y=I(success/total), x=x)) + geom_ribbon(aes(ymin=lower, ymax=upper), fill='blue', alpha=0.2) + geom_line() + scale_y_continuous('Probability of success')+ theme_classic()+ theme(legend.key.width=unit(2,'lines'), legend.position=c(0,1), legend.justification=c(0,1), axis.title.y=element_text(margin(r=2,unit='lines')))
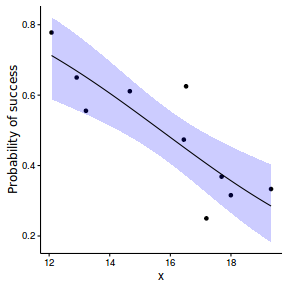
Beta regression (percent cover)
Percent cover (or any percentage for that matter) can logically only range from 0 to 1 (0, 100%). When the observed values are sufficiently away from either 0 and 1, a Gaussian distribution might be effective. However, when observations are close to 0 or 1, Gaussian based models are likely to be poor representations of the true situation at these boundaries. Indeed, predictions close to these boundaries (or the confidence intervals) may actually fall outside the logical range.
The beta distribution is defined for any real number between 0 and 1. The beta distribution is a relatively complex modelling distribution as it can take on a variety of forms. As with models that incorporate a binomial distribution, the beta distribution is typically associated with a logit link function.
- the number of levels of $x$ = 10
- the continuous $x$ variable is a random uniform spread of measurements between 10 and 20
- the rate of change in log odds ratio per unit change in x (slope) = -0.8. The magnitude of the slope is an indicator of how abruptly the log odds ratio changes. A very abrupt change would occur if there was very little variability in the trend. That is, a large slope would be indicative of a threshold effect. A lower slope indicates a gradual change in odds ratio and thus a less obvious and precise switch between the likelihood of 1 vs 0.
- the inflection point (point where the slope of the line is greatest) = 16. The inflection point indicates the value of the predictor ($x$) where the log Odds are 50% (switching between 1 being more likely and 0 being more likely). This is also known as the LD50.
- the intercept (which has no real interpretation) is equal to the negative of the slope multiplied by the inflection point
- generate the values of $y$ expected at each $x$ value, we evaluate the linear predictor.
- generate percentage cover observations by drawing values from a beta distribution with shape parameters defined according to: $$ \begin{align} \text{shape1} (\alpha) &=\Bigg(\frac{1-\mu}{\sigma^2} - \frac{1}{\mu}\Bigg)\\ \text{shape2} (\beta) &=\alpha\Bigg(\frac{1}{\mu} - 1 \Bigg)\\ \end{align} $$ these cover values are expressed as proportions rather than percentages since values need to be between 0 and 1. The mean ($\mu$) is determined by the linear predictor and the variance ($\sigma^2) we will nominate as 0.01.
set.seed(8) #The number of levels of x n.x <- 10 #Create x values that at uniformly distributed throughout the rate of 10 to 20 x <- sort(runif(n = n.x, min = 10, max =20)) slope=-.8 #Inflection point is where the slope of the line is greatest #this is also the LD50 point inflect <- 16 #Intercept (no interpretation) intercept <- -1*(slope*inflect) #The linear predictor linpred <- intercept+slope*x y.pred <- exp(linpred)/(1+exp(linpred)) sigma=0.01 estBetaParams <- function(mu, var) { alpha <- ((1 - mu) / var - 1 / mu) * mu ^ 2 beta <- alpha * (1 / mu - 1) return(params = list(alpha = alpha, beta = beta)) } betaShapes=estBetaParams(y.pred, sigma) y=rbeta(n=n.x, shape1=betaShapes$alpha, shape2=betaShapes$beta) dat <- data.frame(y,x)
$$ \begin{align} log\left(\frac{\pi}{1-\pi}\right)&=\beta_0+\beta_1x_1+\epsilon_i, \hspace{1cm}\epsilon\sim Beta(\alpha, \beta)\\ \alpha &=\Bigg(\frac{1-\mu}{\sigma^2} - \frac{1}{\mu}\Bigg)\\ \beta &=\alpha\Bigg(\frac{1}{\mu} - 1 \Bigg)\\ \end{align} $$
library(betareg) dat.glm <- betareg(y~x, data=dat) summary(dat.glm)
Call:
betareg(formula = y ~ x, data = dat)
Standardized weighted residuals 2:
Min 1Q Median 3Q Max
-2.9455 -0.9286 -0.0994 0.9147 1.3906
Coefficients (mean model with logit link):
Estimate Std. Error z value Pr(>|z|)
(Intercept) 11.7680 2.0298 5.798 6.72e-09 ***
x -0.7634 0.1281 -5.957 2.57e-09 ***
Phi coefficients (precision model with identity link):
Estimate Std. Error z value Pr(>|z|)
(phi) 9.427 4.278 2.204 0.0275 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Type of estimator: ML (maximum likelihood)
Log-likelihood: 12.96 on 3 Df
Pseudo R-squared: 0.6214
Number of iterations: 32 (BFGS) + 8 (Fisher scoring)
modelString=" model{ for (i in 1:N) { y[i] ~ dbeta(a[i],b[i]) a[i] <- eta[i] * phi b[i] <- (1 - eta[i]) * phi logit(eta[i]) <- beta0+beta1*x[i] } beta0 ~ dnorm(0,1.0E-06) beta1 ~ dnorm(0,1.0E-06) phi ~ dgamma(0.01, 0.01) } " library(R2jags) dat.list <- with(dat, list(y=y, x=x, N=nrow(dat))) dat.jags <- jags(data=dat.list,model.file=textConnection(modelString), param=c('beta0','beta1','phi'), n.chains=3, n.iter=20000, n.burnin=10000, n.thin=100)
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 88 Initializing model
print(dat.jags)
Inference for Bugs model at "5", fit using jags,
3 chains, each with 20000 iterations (first 10000 discarded), n.thin = 100
n.sims = 300 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta0 11.783 2.378 7.636 10.183 11.458 13.363 16.476 1.020 120
beta1 -0.764 0.143 -1.052 -0.856 -0.753 -0.668 -0.500 1.026 90
phi 8.832 4.228 2.579 5.747 8.345 11.059 19.175 1.002 300
deviance -22.691 2.689 -25.702 -24.548 -23.459 -21.564 -15.303 1.025 250
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 3.6 and DIC = -19.1
DIC is an estimate of expected predictive error (lower deviance is better).
library(rstan) modelString=" data { int<lower=1> n; // number of observations vector[n] y; // response variable int<lower=1> nX; // number of fixed effects matrix[n, nX] X; // FE design matrix } transformed data { } parameters { vector[nX] beta; // fixed effects real<lower=0> phi; // precision parameter } transformed parameters { vector[n] eta; // linear predictor // compute linear predictor eta <- X * beta; for (i in 1:n) { eta[i] <- inv_logit(eta[i]); } } model { // prior specifications phi ~ gamma(0.01, 0.01); beta ~ normal(0, 1000); // likelihood contribution y ~ beta(eta * phi, (1 - eta) * phi); } generated quantities { } " Xmat <- model.matrix(~x,data=dat) dat.list <- with(dat, list(y = y, X = Xmat,nX=ncol(Xmat),n = nrow(dat))) library(rstan) dat.rstan <- stan(data=dat.list, model_code=modelString, chains=3, iter=1000, warmup=500, thin=2, save_dso=TRUE )
SAMPLING FOR MODEL 'd261ea666f0b7a9209e5feedf9d26f12' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.250474 seconds (Warm-up) # 0.133006 seconds (Sampling) # 0.38348 seconds (Total) # SAMPLING FOR MODEL 'd261ea666f0b7a9209e5feedf9d26f12' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.255301 seconds (Warm-up) # 0.200749 seconds (Sampling) # 0.45605 seconds (Total) # SAMPLING FOR MODEL 'd261ea666f0b7a9209e5feedf9d26f12' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.292455 seconds (Warm-up) # 0.172565 seconds (Sampling) # 0.46502 seconds (Total) #
print(dat.rstan, pars=c('beta','phi'))
Inference for Stan model: d261ea666f0b7a9209e5feedf9d26f12.
3 chains, each with iter=1000; warmup=500; thin=2;
post-warmup draws per chain=250, total post-warmup draws=750.
mean se_mean sd 2.5% 25% 50% 75% 97.5% n_eff Rhat
beta[1] 11.72 0.15 2.47 6.40 10.14 11.69 13.35 16.37 289 1.01
beta[2] -0.76 0.01 0.15 -1.05 -0.86 -0.76 -0.67 -0.43 283 1.01
phi 7.86 0.17 3.02 2.55 5.72 7.62 9.81 14.15 321 1.00
Samples were drawn using NUTS(diag_e) at Mon Feb 22 21:30:57 2016.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).
library(brms) dat.brm <- brm(y~x, data=dat, family='beta', prior=c(set_prior('normal(0,1000)', class='b')), n.chains=3, n.iter=1000, warmup=500, n.thin=2 )
SAMPLING FOR MODEL 'beta(logit) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 7.54483 seconds (Warm-up) # 17.3168 seconds (Sampling) # 24.8616 seconds (Total) # SAMPLING FOR MODEL 'beta(logit) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.112244 seconds (Warm-up) # 0.056446 seconds (Sampling) # 0.16869 seconds (Total) # SAMPLING FOR MODEL 'beta(logit) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.085228 seconds (Warm-up) # 0.061105 seconds (Sampling) # 0.146333 seconds (Total) #
summary(dat.brm)
Family: beta (logit)
Formula: y ~ x
Data: dat (Number of observations: 10)
Samples: 3 chains, each with iter = 1000; warmup = 500; thin = 2;
total post-warmup samples = 750
WAIC: Not computed
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
Intercept 13.43 3.26 7.56 18.08 2 1.77
x -0.86 0.19 -1.13 -0.50 2 1.70
Family Specific Parameters:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
phi 19.82 17.26 3.08 49.04 2 5.94
Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
is a crude measure of effective sample size, and Rhat is the potential
scale reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
}
data {
int<lower=1> N; // number of observations
vector[N] Y; // response variable
int<lower=1> K; // number of fixed effects
matrix[N, K] X; // FE design matrix
vector[K] X_means; // column means of X
}
transformed data {
}
parameters {
real temp_Intercept; // temporary Intercept
vector[K] b; // fixed effects
real<lower=0> phi; // precision parameter
}
transformed parameters {
vector[N] eta; // linear predictor
// compute linear predictor
eta <- X * b + temp_Intercept;
for (n in 1:N) {
eta[n] <- inv_logit(eta[n]);
}
}
model {
// prior specifications
b ~ normal(0,1000);
phi ~ gamma(0.01, 0.01);
// likelihood contribution
Y ~ beta(eta * phi, (1 - eta) * phi);
}
generated quantities {
real b_Intercept; // fixed effects intercept
b_Intercept <- temp_Intercept - dot_product(X_means, b);
}
fitted <- subset(dat, select=c(x,y)) fitted$y <- NA newdata <- expand.grid(x=seq(min(dat$x), max(dat$x), len=100), y=NA) dat.pred <- rbind(dat,fitted,newdata)
Now lets fit the model.
#fit the model library(INLA) dat.inla <- inla(y~x, family='beta', data=dat.pred, control.family=list(link='logit'), control.predictor=list(link=1, compute=TRUE), control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE)) #examine the regular summary summary(dat.inla)
Call:
c("inla(formula = y ~ x, family = \"beta\", data = dat.pred, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE), control.predictor = list(link = 1, ", " compute = TRUE), control.family = list(link = \"logit\"))" )
Time used:
Pre-processing Running inla Post-processing Total
0.0695 0.0710 0.0248 0.1653
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 11.6845 2.0768 7.7662 11.6131 15.9882 11.4692 0
x -0.7599 0.1252 -1.0177 -0.7562 -0.5220 -0.7488 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
precision parameter for the beta observations 8.711 3.662 3.357 8.142 17.46 6.989
Expected number of effective parameters(std dev): 2.00(0.00)
Number of equivalent replicates : 5.00
Deviance Information Criterion (DIC) ...: -21.16
Effective number of parameters .........: 2.456
Watanabe-Akaike information criterion (WAIC) ...: -20.19
Effective number of parameters .................: 2.797
Marginal log-Likelihood: 5.913
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
newdata <- cbind(newdata, dat.inla$summary.fitted.values[(nrow(dat)+nrow(fitted)+1):nrow(dat.pred),]) head(newdata)
x y mean sd 0.025quant 0.5quant 0.975quant mode fitted.predictor.021 12.07823 NA 0.9140861 0.04629234 0.7996400 0.9228020 0.9775402 0.9387765 fitted.predictor.022 12.15141 NA 0.9100518 0.04750029 0.7931865 0.9187774 0.9758870 0.9348454 fitted.predictor.023 12.22459 NA 0.9058414 0.04871891 0.7865733 0.9145631 0.9741167 0.9307015 fitted.predictor.024 12.29776 NA 0.9014486 0.04994645 0.7797996 0.9101522 0.9722216 0.9263219 fitted.predictor.025 12.37094 NA 0.8968674 0.05118101 0.7728648 0.9055379 0.9701937 0.9217106 fitted.predictor.026 12.44412 NA 0.8920916 0.05242054 0.7657683 0.9007135 0.9680244 0.9168501
newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) library(grid) ggplot(newdata, aes(y=mean*100, x=x)) + geom_blank()+ geom_point(data=dat, aes(y=y*100, x=x)) + geom_ribbon(aes(ymin=lower*100, ymax=upper*100), fill='blue', alpha=0.2) + geom_line() + scale_y_continuous(expression(Cover~abundance~('%')))+ theme_classic()+ theme(legend.key.width=unit(2,'lines'), legend.position=c(0,1), legend.justification=c(0,1))
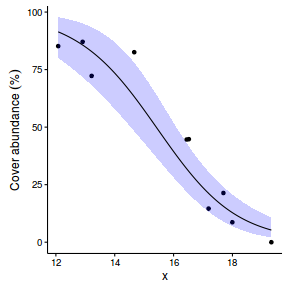
Poisson regression (count data)
Lets say we wanted to model the abundance of an item ($y$) against a continuous predictor ($x$). As this section is mainly about the generation of artificial data (and not specifically about what to do with the data), understanding the actual details are optional and can be safely skipped. Consequently, I have folded (toggled) this section away.
- the sample size = 20
- the continuous $x$ variable is a random uniform spread of measurements between 1 and 20
- the rate of change in log $y$ per unit of $x$ (slope) = 0.1.
- the value of $x$ when log$y$ equals 0 (when $y$=1)
- to generate the values of $y$ expected at each $x$ value, we evaluate the linear predictor (created by calculating the outer product of the model matrix and the regression parameters). These expected values are then transformed into a scale mapped by (0,$\infty$) by using the log function $e^{linear~predictor}$
- finally, we generate $y$ values by using the expected $y$ values ($\lambda$) as probabilities when drawing random numbers from a Poisson distribution. This step adds random noise to the expected $y$ values and returns only 0's and positive integers.
set.seed(8) #The number of samples n.x <- 20 #Create x values that at uniformly distributed throughout the rate of 1 to 20 x <- sort(runif(n = n.x, min = 1, max =20)) mm <- model.matrix(~x) intercept <- 0.6 slope=0.1 #The linear predictor linpred <- mm %*% c(intercept,slope) #Predicted y values lambda <- exp(linpred) #Add some noise and make binomial y <- rpois(n=n.x, lambda=lambda) dat <- data.frame(y,x) data.pois <- dat
$$ \begin{align} y_i &\sim{} Pois(\lambda_i)\\ log(\lambda_i)&=\beta_0+\beta_1x_1\\ \end{align} $$
dat.glm <- glm(y~x, data=data.pois, family="poisson") summary(dat.glm)
Call:
glm(formula = y ~ x, family = "poisson", data = data.pois)
Deviance Residuals:
Min 1Q Median 3Q Max
-1.6353 -0.7049 0.0440 0.5624 2.0457
Coefficients:
Estimate Std. Error z value Pr(>|z|)
(Intercept) 0.56002 0.25395 2.205 0.0274 *
x 0.11151 0.01858 6.000 1.97e-09 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
(Dispersion parameter for poisson family taken to be 1)
Null deviance: 55.614 on 19 degrees of freedom
Residual deviance: 17.226 on 18 degrees of freedom
AIC: 90.319
Number of Fisher Scoring iterations: 4
modelString=" model{ for (i in 1:N) { y[i] ~ dpois(lambda[i]) log(lambda[i]) <- max(-20,min(20,beta0+beta1*x[i])) } beta0 ~ dnorm(0,1.0E-06) beta1 ~ dnorm(0,1.0E-06) } " dat.list <- with(data.pois, list(y=y, x=x, N=nrow(data.pois))) dat.jags <- jags(data=dat.list,model.file=textConnection(modelString), param=c('beta0','beta1'), n.chains=3, n.iter=20000, n.burnin=10000, n.thin=100)
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 147 Initializing model
print(dat.jags)
Inference for Bugs model at "5", fit using jags,
3 chains, each with 20000 iterations (first 10000 discarded), n.thin = 100
n.sims = 300 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta0 0.556 0.246 0.077 0.407 0.533 0.718 1.088 1.009 300
beta1 0.111 0.018 0.073 0.099 0.111 0.124 0.143 1.007 300
deviance 88.241 1.734 86.376 86.873 87.781 89.023 92.674 1.000 300
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 1.5 and DIC = 89.8
DIC is an estimate of expected predictive error (lower deviance is better).
library(rstan) modelString=" data { int<lower=1> n; int<lower=1> nX; int y[n]; matrix [n,nX] X; } parameters { vector [nX] beta; } transformed parameters { vector [n] mu; mu<-X*beta; } model { beta ~ normal(0,1000); y ~ poisson_log(mu); } " Xmat <- model.matrix(~x,data=data.pois) dat.list <- with(data.pois, list(y = y, X = Xmat, nX=ncol(Xmat),n = nrow(data.pois))) library(rstan) dat.rstan <- stan(data=dat.list, model_code=modelString, chains=3, iter=1000, warmup=500, thin=2, save_dso=TRUE )
SAMPLING FOR MODEL '95c0e3e05517b98739c4f0c17ec17bdd' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.086074 seconds (Warm-up) # 0.040789 seconds (Sampling) # 0.126863 seconds (Total) # SAMPLING FOR MODEL '95c0e3e05517b98739c4f0c17ec17bdd' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.051455 seconds (Warm-up) # 0.030687 seconds (Sampling) # 0.082142 seconds (Total) # SAMPLING FOR MODEL '95c0e3e05517b98739c4f0c17ec17bdd' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.068899 seconds (Warm-up) # 0.036493 seconds (Sampling) # 0.105392 seconds (Total) #
print(dat.rstan, pars=c('beta'))
Inference for Stan model: 95c0e3e05517b98739c4f0c17ec17bdd.
3 chains, each with iter=1000; warmup=500; thin=2;
post-warmup draws per chain=250, total post-warmup draws=750.
mean se_mean sd 2.5% 25% 50% 75% 97.5% n_eff Rhat
beta[1] 0.54 0.01 0.24 0.05 0.39 0.55 0.70 1.01 255 1.01
beta[2] 0.11 0.00 0.02 0.08 0.10 0.11 0.12 0.15 239 1.01
Samples were drawn using NUTS(diag_e) at Tue Feb 23 11:04:58 2016.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).
library(brms) dat.brm <- brm(y~x, data=data.pois, family='poisson', prior=c(set_prior('normal(0,1000)', class='b')), n.chains=3, n.iter=1000, warmup=500, n.thin=2 )
SAMPLING FOR MODEL 'poisson(log) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.021825 seconds (Warm-up) # 0.01908 seconds (Sampling) # 0.040905 seconds (Total) # SAMPLING FOR MODEL 'poisson(log) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.03022 seconds (Warm-up) # 0.028595 seconds (Sampling) # 0.058815 seconds (Total) # SAMPLING FOR MODEL 'poisson(log) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.021021 seconds (Warm-up) # 0.018216 seconds (Sampling) # 0.039237 seconds (Total) #
summary(dat.brm)
Family: poisson (log)
Formula: y ~ x
Data: data.pois (Number of observations: 20)
Samples: 3 chains, each with iter = 1000; warmup = 500; thin = 2;
total post-warmup samples = 750
WAIC: Not computed
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
Intercept 0.54 0.26 -0.02 1.00 305 1.02
x 0.11 0.02 0.08 0.15 362 1.02
Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
is a crude measure of effective sample size, and Rhat is the potential
scale reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
}
data {
int<lower=1> N; // number of observations
int Y[N]; // response variable
int<lower=1> K; // number of fixed effects
matrix[N, K] X; // FE design matrix
vector[K] X_means; // column means of X
}
transformed data {
}
parameters {
real temp_Intercept; // temporary Intercept
vector[K] b; // fixed effects
}
transformed parameters {
vector[N] eta; // linear predictor
// compute linear predictor
eta <- X * b + temp_Intercept;
}
model {
// prior specifications
b ~ normal(0,1000);
// likelihood contribution
Y ~ poisson_log(eta);
}
generated quantities {
real b_Intercept; // fixed effects intercept
b_Intercept <- temp_Intercept - dot_product(X_means, b);
}
fitted <- subset(dat, select=c(x,y)) fitted$y <- NA newdata <- expand.grid(x=seq(min(data.pois$x), max(data.pois$x), len=100), y=NA) dat.pred <- rbind(dat,fitted,newdata)
Now lets fit the model.
#fit the model dat.inla <- inla(y~x, family='poisson', data=dat.pred, control.family=list(link='log'), control.predictor=list(link=1, compute=TRUE), control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE)) #examine the regular summary summary(dat.inla)
Call:
c("inla(formula = y ~ x, family = \"poisson\", data = dat.pred, control.compute = list(dic = TRUE, ", " cpo = TRUE, waic = TRUE), control.predictor = list(link = 1, ", " compute = TRUE), control.family = list(link = \"log\"))" )
Time used:
Pre-processing Running inla Post-processing Total
0.0898 0.0448 0.0357 0.1704
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 0.5624 0.2540 0.0469 0.5683 1.0450 0.5803 0
x 0.1115 0.0186 0.0755 0.1114 0.1484 0.1111 0
The model has no random effects
The model has no hyperparameters
Expected number of effective parameters(std dev): 2.002(0.00)
Number of equivalent replicates : 9.991
Deviance Information Criterion (DIC) ...: 90.31
Effective number of parameters .........: 2.00
Watanabe-Akaike information criterion (WAIC) ...: 90.32
Effective number of parameters .................: 1.809
Marginal log-Likelihood: -52.09
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
newdata <- cbind(newdata, dat.inla$summary.fitted.values[(nrow(dat)+nrow(fitted)+1):nrow(dat.pred),]) head(newdata)
x y mean sd 0.025quant 0.5quant 0.975quant mode fitted.predictor.041 1.024733 NA 2.021898 0.4765762 1.217135 1.978066 3.077859 1.893767 fitted.predictor.042 1.203403 NA 2.061043 0.4793782 1.249221 2.017707 3.121132 1.934330 fitted.predictor.043 1.382074 NA 2.100965 0.4821355 1.282138 2.058139 3.165053 1.975705 fitted.predictor.044 1.560745 NA 2.141682 0.4848464 1.315907 2.099378 3.209754 2.017948 fitted.predictor.045 1.739415 NA 2.183208 0.4875087 1.350549 2.141438 3.255151 2.061031 fitted.predictor.046 1.918086 NA 2.225560 0.4901204 1.386083 2.184338 3.301238 2.104974
newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) library(grid) ggplot(newdata, aes(y=mean, x=x)) + geom_blank()+ geom_point(data=data.pois, aes(y=y, x=x)) + geom_ribbon(aes(ymin=lower, ymax=upper), fill='blue', alpha=0.2) + geom_line() + theme_classic()+ theme(legend.key.width=unit(2,'lines'), legend.position=c(0,1), legend.justification=c(0,1))
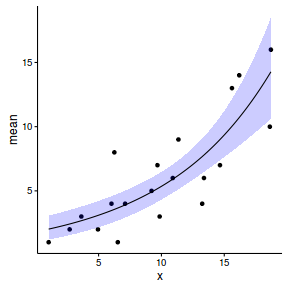
Poisson regression (count data) with overdispersion
There are numerous ways to deal with overdispersion. The traditional way was to use a 'quasi' family. Such an approach fits a regular distribution and then alters the standard errors according to the degree of overdispersion and is considered by many to be a bit of a hack.
If overdispersion is the consequence of latent (un-measured or un-modelled) influences inflating the variances, then one alternative is to incorporate a latent variable that will 'soak' up this additional variance. This is the theory behind including a unit-level random effect.
- define a function that will generate random samples from a quasi-poisson distribution $$QuasiPoisson\left(\frac{\mu}{\phi-1}, \frac{1}{\phi}\right)$$ where $\mu$ is the expected value (mean) and $\phi$ is the dispersion factor such that $var(y)=\phi\mu$.
- the sample size = 20
- the continuous $x$ variable is a random uniform spread of measurements between 1 and 20
- the rate of change in log $y$ per unit of $x$ (slope) = 0.1.
- the value of $x$ when log$y$ equals 0 (when $y$=1)
- to generate the values of $y$ expected at each $x$ value, we evaluate the linear predictor (created by calculating the outer product of the model matrix and the regression parameters). These expected values are then transformed into a scale mapped by (0,$\infty$) by using the log function $e^{linear~predictor}$
- finally, we generate $y$ values by using the expected $y$ values ($\lambda$) as probabilities when drawing random numbers from a quasi-poisson distribution. This step adds random noise to the expected $y$ values and returns only 0's and positive integers.
#Quasi-poisson distrition rqpois <- function(n, lambda, phi) { mu = lambda r = rnbinom(n, size=mu/phi/(1-1/phi),prob=1/phi) return(r) } set.seed(8) #The number of samples n.x <- 20 #Create x values that at uniformly distributed throughout the rate of 1 to 20 x <- sort(runif(n = n.x, min = 1, max =20)) mm <- model.matrix(~x) intercept <- 0.6 slope=0.1 #The linear predictor linpred <- mm %*% c(intercept,slope) #Predicted y values lambda <- exp(linpred) #Add some noise and make binomial y <- rqpois(n=n.x, lambda=lambda, phi=4) dat.qp <- data.frame(y,x) data.qp <- dat.qp
$$ \begin{align} y_{ij} &\sim{} Pois(\lambda_{ij})\\ log(\lambda_{ij})&=\beta_0+\beta_1x_1 + \sum^j_{J=1} \gamma U_j\\ \end{align} $$
library(lme4) data.qp$unit=1:nrow(data.qp) dat.glm <- glmer(y~x+(1|unit), data=data.qp, family="poisson") summary(dat.glm)
Generalized linear mixed model fit by maximum likelihood (Laplace Approximation) ['glmerMod']
Family: poisson ( log )
Formula: y ~ x + (1 | unit)
Data: data.qp
AIC BIC logLik deviance df.resid
114.6 117.6 -54.3 108.6 17
Scaled residuals:
Min 1Q Median 3Q Max
-1.37873 -0.65032 0.04504 0.41908 0.68899
Random effects:
Groups Name Variance Std.Dev.
unit (Intercept) 0.4251 0.652
Number of obs: 20, groups: unit, 20
Fixed effects:
Estimate Std. Error z value Pr(>|z|)
(Intercept) 0.19971 0.47627 0.419 0.674981
x 0.12684 0.03833 3.309 0.000936 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Correlation of Fixed Effects:
(Intr)
x -0.915
data.qp$unit=1:nrow(data.qp) modelString=" model{ for (i in 1:N) { y[i] ~ dpois(lambda[i]) log(lambda[i]) <- max(-20,min(20,beta0+beta1*x[i] + gamma[U[i]])) } beta0 ~ dnorm(0,1.0E-06) beta1 ~ dnorm(0,1.0E-06) for (j in 1:nU) { gamma[j] ~ dnorm(0, tau) } ## Half-cauchy (scale=25) prior tau <- pow(sigma,-2) sigma <-z/sqrt(chSq) z ~ dnorm(0, .0016)I(0,) chSq ~ dgamma(0.5, 0.5) } " dat.list <- with(data.qp, list(y=y, x=x, U=as.numeric(unit), nU=nrow(data.qp), N=nrow(data.qp))) dat.jags <- jags(data=dat.list,model.file=textConnection(modelString), param=c('beta0','beta1','sigma'), n.chains=3, n.iter=20000, n.burnin=10000, n.thin=100)
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 197 Initializing model
print(dat.jags)
Inference for Bugs model at "5", fit using jags,
3 chains, each with 20000 iterations (first 10000 discarded), n.thin = 100
n.sims = 300 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta0 0.001 0.651 -1.431 -0.362 0.075 0.421 1.201 1.047 60
beta1 0.141 0.053 0.046 0.110 0.137 0.172 0.250 1.049 67
sigma 0.852 0.284 0.456 0.666 0.802 0.965 1.576 1.006 210
deviance 84.483 6.824 74.009 79.661 84.080 88.029 98.306 0.997 300
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 23.4 and DIC = 107.9
DIC is an estimate of expected predictive error (lower deviance is better).
data.qp$unit=1:nrow(data.qp) library(rstan) modelString=" data { int<lower=1> n; int<lower=1> nX; int<lower=1> nU; int y[n]; matrix [n,nX] X; int U[n]; } parameters { vector [nX] beta; vector [nU] gamma; real<lower=0> sigma; } transformed parameters { vector [n] mu; mu<-X*beta; for (i in 1:n) { mu[i] <- mu[i] + gamma[U[i]]; } } model { beta ~ normal(0,1000); sigma ~ cauchy(0,25); gamma ~ normal(0,sigma); y ~ poisson_log(mu); } " Xmat <- model.matrix(~x,data=data.qp) dat.list <- with(data.qp, list(y = y, X = Xmat, nX=ncol(Xmat), U=as.numeric(unit), nU=nrow(data.qp), n = nrow(data.qp))) library(rstan) dat.rstan <- stan(data=dat.list, model_code=modelString, chains=3, iter=1000, warmup=500, thin=2, save_dso=TRUE )
SAMPLING FOR MODEL '6b01137f7762e83c8564fa8b7bbd08b8' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.291649 seconds (Warm-up) # 0.151107 seconds (Sampling) # 0.442756 seconds (Total) # SAMPLING FOR MODEL '6b01137f7762e83c8564fa8b7bbd08b8' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.279321 seconds (Warm-up) # 0.102494 seconds (Sampling) # 0.381815 seconds (Total) # SAMPLING FOR MODEL '6b01137f7762e83c8564fa8b7bbd08b8' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.306079 seconds (Warm-up) # 0.110866 seconds (Sampling) # 0.416945 seconds (Total) #
print(dat.rstan, pars=c('beta','sigma'))
Inference for Stan model: 6b01137f7762e83c8564fa8b7bbd08b8.
3 chains, each with iter=1000; warmup=500; thin=2;
post-warmup draws per chain=250, total post-warmup draws=750.
mean se_mean sd 2.5% 25% 50% 75% 97.5% n_eff Rhat
beta[1] 0.01 0.03 0.59 -1.25 -0.36 0.06 0.42 1.01 314 1.00
beta[2] 0.14 0.00 0.05 0.05 0.11 0.13 0.17 0.23 287 1.00
sigma 0.85 0.02 0.26 0.47 0.67 0.81 0.98 1.44 248 1.01
Samples were drawn using NUTS(diag_e) at Tue Feb 23 12:30:49 2016.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).
data.qp$unit=1:nrow(data.qp) library(brms) dat.brm <- brm(y~x+(1|unit), data=data.qp, family='poisson', prior=c(set_prior('normal(0,1000)', class='b')), chains=3, iter=1000, warmup=500, thin=2 )
SAMPLING FOR MODEL 'poisson(log) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.139169 seconds (Warm-up) # 0.059387 seconds (Sampling) # 0.198556 seconds (Total) # SAMPLING FOR MODEL 'poisson(log) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.165589 seconds (Warm-up) # 0.062659 seconds (Sampling) # 0.228248 seconds (Total) # SAMPLING FOR MODEL 'poisson(log) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.140647 seconds (Warm-up) # 0.056132 seconds (Sampling) # 0.196779 seconds (Total) #
summary(dat.brm)
Family: poisson (log)
Formula: y ~ x + (1 | unit)
Data: data.qp (Number of observations: 20)
Samples: 3 chains, each with iter = 1000; warmup = 500; thin = 2;
total post-warmup samples = 750
WAIC: Not computed
Random Effects:
~unit (Number of levels: 20)
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
sd(Intercept) 0.83 0.26 0.46 1.43 171 1.01
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
Intercept 0.07 0.55 -1.04 1.14 480 1
x 0.13 0.05 0.05 0.23 348 1
Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
is a crude measure of effective sample size, and Rhat is the potential
scale reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
}
data {
int<lower=1> N; // number of observations
int Y[N]; // response variable
int<lower=1> K; // number of fixed effects
matrix[N, K] X; // FE design matrix
vector[K] X_means; // column means of X
// data for random effects of unit
int<lower=1> J_1[N]; // RE levels
int<lower=1> N_1; // number of levels
int<lower=1> K_1; // number of REs
real Z_1[N]; // RE design matrix
}
transformed data {
}
parameters {
real temp_Intercept; // temporary Intercept
vector[K] b; // fixed effects
vector[N_1] pre_1; // unscaled REs
real<lower=0> sd_1; // RE standard deviation
}
transformed parameters {
vector[N] eta; // linear predictor
vector[N_1] r_1; // REs
// compute linear predictor
eta <- X * b + temp_Intercept;
r_1 <- sd_1 * (pre_1); // scale REs
for (n in 1:N) {
eta[n] <- eta[n] + Z_1[n] * r_1[J_1[n]];
}
}
model {
// prior specifications
b ~ normal(0,1000);
sd_1 ~ student_t(3, 0, 5);
pre_1 ~ normal(0, 1);
// likelihood contribution
Y ~ poisson_log(eta);
}
generated quantities {
real b_Intercept; // fixed effects intercept
b_Intercept <- temp_Intercept - dot_product(X_means, b);
}
fitted <- subset(data.qp, select=c(x,y, unit)) fitted$y <- NA newdata <- expand.grid(x=seq(min(data.qp$x), max(data.qp$x), len=100), y=NA, unit=NA) dat.pred <- rbind(data.qp,fitted,newdata)
Now lets fit the model.
#fit the model dat.inla <- inla(y~x+f(unit, model='iid'), family='poisson', data=dat.pred, control.family=list(link='log'), control.predictor=list(link=1, compute=TRUE), control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE)) #examine the regular summary summary(dat.inla)
Call:
c("inla(formula = y ~ x + f(unit, model = \"iid\"), family = \"poisson\", ", " data = dat.pred, control.compute = list(dic = TRUE, cpo = TRUE, ", " waic = TRUE), control.predictor = list(link = 1, compute = TRUE), ", " control.family = list(link = \"log\"))")
Time used:
Pre-processing Running inla Post-processing Total
0.0950 0.0728 0.0331 0.2008
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 0.2176 0.4647 -0.7614 0.2394 1.0738 0.2822 0
x 0.1263 0.0378 0.0549 0.1251 0.2046 0.1229 0
Random effects:
Name Model
unit IID model
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
Precision for unit 3.154 2.022 0.8659 2.647 8.429 1.889
Expected number of effective parameters(std dev): 13.15(1.516)
Number of equivalent replicates : 1.521
Deviance Information Criterion (DIC) ...: 100.91
Effective number of parameters .........: 13.48
Watanabe-Akaike information criterion (WAIC) ...: 100.97
Effective number of parameters .................: 10.12
Marginal log-Likelihood: -70.59
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
newdata <- cbind(newdata, dat.inla$summary.fitted.values[(nrow(data.qp)+nrow(fitted)+1):nrow(dat.pred),]) head(newdata)
x y unit mean sd 0.025quant 0.5quant 0.975quant mode fitted.predictor.041 1.024733 NA NA 1.546157 0.6600472 0.5723928 1.443850 3.115993 1.263051 fitted.predictor.042 1.203403 NA NA 1.577515 0.6633672 0.5928836 1.476424 3.151031 1.297411 fitted.predictor.043 1.382074 NA NA 1.609575 0.6666484 0.6140817 1.509731 3.186584 1.332625 fitted.predictor.044 1.560745 NA NA 1.642354 0.6698890 0.6360093 1.543786 3.222667 1.368681 fitted.predictor.045 1.739415 NA NA 1.675869 0.6730872 0.6586892 1.578607 3.259294 1.405544 fitted.predictor.046 1.918086 NA NA 1.710138 0.6762414 0.6821445 1.614211 3.296481 1.443231
newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) library(grid) ggplot(newdata, aes(y=mean, x=x)) + geom_blank()+ geom_point(data=data.qp, aes(y=y, x=x)) + geom_ribbon(aes(ymin=lower, ymax=upper), fill='blue', alpha=0.2) + geom_line() + theme_classic()+ theme(legend.key.width=unit(2,'lines'), legend.position=c(0,1), legend.justification=c(0,1))
Negative Binomial regression (overdispersed count data)
The Negative Binomial distribution strictly represents the number of successes before a failure. In practice it is a beta-poisson distribution. As such, it is a distribution that is defined by two parameters (which can be defined in terms of location and scale). When the model has a dispersion parameter of 1 (when $\lambda = \mu = \sigma^2$), the Negative Binomial distribution is equivalent to a Poisson distribution.
Hence rather than assume that the dispersion parameter is 1 ($\lambda = \mu = \sigma^2$), we can use a distribution that estimates the scale as well as the location parameter.
- the sample size = 20
- the continuous $x$ variable is a random uniform spread of measurements between 1 and 20
- the rate of change in log $y$ per unit of $x$ (slope) = 0.1.
- the value of $x$ when log$y$ equals 0 (when $y$=1)
- to generate the values of $y$ expected at each $x$ value, we evaluate the linear predictor (created by calculating the outer product of the model matrix and the regression parameters). These expected values are then transformed into a scale mapped by (0,$\infty$) by using the log function $e^{linear~predictor}$
- finally, we generate $y$ values by using the expected $y$ values ($\lambda$) as probabilities when drawing random numbers from a Poisson distribution. This step adds random noise to the expected $y$ values and returns only 0's and positive integers.
set.seed(37) #16 #35 #The number of samples n.x <- 20 #Create x values that at uniformly distributed throughout the rate of 1 to 20 x <- sort(runif(n = n.x, min = 1, max =20)) mm <- model.matrix(~x) intercept <- 0.6 slope=0.1 #The linear predictor linpred <- mm %*% c(intercept,slope) #Predicted y values lambda <- exp(linpred) #Add some noise and make binomial y <- rnbinom(n=n.x, mu=lambda, size=1) dat.nb <- data.frame(y,x) data.nb <- dat.nb
$$ \begin{align} y_{ij} &\sim{} NB(p_{ij}, size)\\ p_{ij} &= size/(size+\lambda_{ij})\\ log(\lambda_{ij})&=\beta_0+\beta_1x_1 + \sum^j_{J=1} \gamma U_j\\ size &\sim{} U(0.001, 10000)\\ \end{align} $$
library(MASS) dat.glm <- glm.nb(y~x, data=data.nb) summary(dat.glm)
Call:
glm.nb(formula = y ~ x, data = data.nb, init.theta = 2.359878187,
link = log)
Deviance Residuals:
Min 1Q Median 3Q Max
-2.7874 -0.8940 -0.3172 0.4420 1.3660
Coefficients:
Estimate Std. Error z value Pr(>|z|)
(Intercept) 0.74543 0.42534 1.753 0.07968 .
x 0.09494 0.03441 2.759 0.00579 **
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
(Dispersion parameter for Negative Binomial(2.3599) family taken to be 1)
Null deviance: 30.443 on 19 degrees of freedom
Residual deviance: 21.806 on 18 degrees of freedom
AIC: 115.85
Number of Fisher Scoring iterations: 1
Theta: 2.36
Std. Err.: 1.10
2 x log-likelihood: -109.849
data.qp$unit=1:nrow(data.qp) modelString=" model{ for (i in 1:N) { y[i] ~ dnegbin(p[i],size) p[i] <- size/(size+lambda[i]) log(lambda[i]) <- max(-20,min(20,beta0+beta1*x[i])) } beta0 ~ dnorm(0,1.0E-06) beta1 ~ dnorm(0,1.0E-06) size ~ dunif(0.001, 1000) } " dat.list <- with(data.nb, list(y=y, x=x, N=nrow(data.nb))) dat.jags <- jags(data=dat.list,model.file=textConnection(modelString), param=c('beta0','beta1','size'), n.chains=3, n.iter=20000, n.burnin=10000, n.thin=100)
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 190 Initializing model
print(dat.jags)
Inference for Bugs model at "5", fit using jags,
3 chains, each with 20000 iterations (first 10000 discarded), n.thin = 100
n.sims = 300 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta0 0.761 0.425 0.014 0.471 0.732 1.014 1.616 0.997 300
beta1 0.094 0.033 0.022 0.073 0.095 0.117 0.150 0.999 300
size 3.916 7.867 1.038 2.034 2.742 3.766 8.598 1.060 160
deviance 113.293 3.196 110.049 111.142 112.441 114.103 121.912 1.043 160
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 5.1 and DIC = 118.4
DIC is an estimate of expected predictive error (lower deviance is better).
library(rstan) modelString=" data { int<lower=1> n; int<lower=1> nX; int y[n]; matrix [n,nX] X; } parameters { vector [nX] beta; real<lower=0> shape; } transformed parameters { vector [n] eta; eta<-X*beta; } model { beta ~ normal(0,1000); shape ~ student_t(3,0,5); y ~ neg_binomial_2_log(eta, shape); } " Xmat <- model.matrix(~x,data=data.nb) dat.list <- with(data.nb, list(y = y, X = Xmat, nX=ncol(Xmat), n = nrow(data.nb))) library(rstan) dat.rstan <- stan(data=dat.list, model_code=modelString, chains=3, iter=1000, warmup=500, thin=2, save_dso=TRUE )
SAMPLING FOR MODEL '7ed6b7a1636f285e8c38d5fd272fc919' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 3.8897 seconds (Warm-up) # 0.22708 seconds (Sampling) # 4.11678 seconds (Total) # SAMPLING FOR MODEL '7ed6b7a1636f285e8c38d5fd272fc919' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 6.20803 seconds (Warm-up) # 12.3776 seconds (Sampling) # 18.5856 seconds (Total) # SAMPLING FOR MODEL '7ed6b7a1636f285e8c38d5fd272fc919' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 3.37625 seconds (Warm-up) # 0.126583 seconds (Sampling) # 3.50283 seconds (Total) #
print(dat.rstan, pars=c('beta','shape'))
Inference for Stan model: 7ed6b7a1636f285e8c38d5fd272fc919.
3 chains, each with iter=1000; warmup=500; thin=2;
post-warmup draws per chain=250, total post-warmup draws=750.
mean se_mean sd 2.5% 25% 50% 75% 97.5% n_eff Rhat
beta[1] 0.57 0.21 0.56 -0.40 0.15 0.60 0.97 1.74 7 1.31
beta[2] 0.11 0.01 0.04 0.03 0.08 0.10 0.13 0.18 11 1.25
shape 1.57 0.05 0.31 0.87 1.37 1.59 1.84 1.98 43 1.08
Samples were drawn using NUTS(diag_e) at Tue Feb 23 16:08:26 2016.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).
library(brms) dat.brm <- brm(y~x, data=data.nb, family='negbinomial', prior=c(set_prior('normal(0,1000)', class='b')), chains=3, iter=1000, warmup=500, thin=2 )
SAMPLING FOR MODEL 'negbinomial(log) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 3.63316 seconds (Warm-up) # 12.482 seconds (Sampling) # 16.1151 seconds (Total) # SAMPLING FOR MODEL 'negbinomial(log) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 7.01036 seconds (Warm-up) # 12.4126 seconds (Sampling) # 19.423 seconds (Total) # SAMPLING FOR MODEL 'negbinomial(log) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 5.72697 seconds (Warm-up) # 4.75621 seconds (Sampling) # 10.4832 seconds (Total) #
summary(dat.brm)
Family: negbinomial (log)
Formula: y ~ x
Data: data.nb (Number of observations: 20)
Samples: 3 chains, each with iter = 1000; warmup = 500; thin = 2;
total post-warmup samples = 750
WAIC: Not computed
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
Intercept 0.79 0.48 -0.04 1.91 80 1.02
x 0.09 0.04 0.01 0.17 104 1.01
Family Specific Parameters:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
shape 1.57 0.29 0.91 1.98 89 1.01
Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
is a crude measure of effective sample size, and Rhat is the potential
scale reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
}
data {
int<lower=1> N; // number of observations
int Y[N]; // response variable
int<lower=1> K; // number of fixed effects
matrix[N, K] X; // FE design matrix
vector[K] X_means; // column means of X
}
transformed data {
}
parameters {
real temp_Intercept; // temporary Intercept
vector[K] b; // fixed effects
real<lower=0> shape; // shape parameter
}
transformed parameters {
vector[N] eta; // linear predictor
// compute linear predictor
eta <- X * b + temp_Intercept;
}
model {
// prior specifications
b ~ normal(0,1000);
shape ~ student_t(3, 0, 5);
// likelihood contribution
Y ~ neg_binomial_2_log(eta, shape);
}
generated quantities {
real b_Intercept; // fixed effects intercept
b_Intercept <- temp_Intercept - dot_product(X_means, b);
}
fitted <- subset(data.nb, select=c(x,y)) fitted$y <- NA newdata <- expand.grid(x=seq(min(data.nb$x), max(data.nb$x), len=100), y=NA) dat.pred <- rbind(data.nb,fitted,newdata)
Now lets fit the model.
#fit the model dat.inla <- inla(y~x, family='nbinomial', data=dat.pred, control.family=list(link='log'), control.predictor=list(link=1, compute=TRUE), control.compute=list(dic=TRUE, cpo=TRUE, waic=TRUE)) #examine the regular summary summary(dat.inla)
Call:
c("inla(formula = y ~ x, family = \"nbinomial\", data = dat.pred, ", " control.compute = list(dic = TRUE, cpo = TRUE, waic = TRUE), ", " control.predictor = list(link = 1, compute = TRUE), control.family = list(link = \"log\"))" )
Time used:
Pre-processing Running inla Post-processing Total
0.0866 0.0483 0.0302 0.1650
Fixed effects:
mean sd 0.025quant 0.5quant 0.975quant mode kld
(Intercept) 0.7439 0.4569 -0.1380 0.7379 1.6633 0.7283 0
x 0.0949 0.0368 0.0226 0.0948 0.1680 0.0944 0
The model has no random effects
Model hyperparameters:
mean sd 0.025quant 0.5quant 0.975quant mode
size for the nbinomial observations (overdispersion) 1.859 0.7529 0.794 1.725 3.702 1.485
Expected number of effective parameters(std dev): 2.00(1e-04)
Number of equivalent replicates : 9.998
Deviance Information Criterion (DIC) ...: 115.27
Effective number of parameters .........: 2.474
Watanabe-Akaike information criterion (WAIC) ...: 115.09
Effective number of parameters .................: 2.055
Marginal log-Likelihood: -63.84
CPO and PIT are computed
Posterior marginals for linear predictor and fitted values computed
newdata <- cbind(newdata, dat.inla$summary.fitted.values[(nrow(data.nb)+nrow(fitted)+1):nrow(dat.pred),]) head(newdata)
x y mean sd 0.025quant 0.5quant 0.975quant mode fitted.predictor.041 1.042191 NA 2.546251 1.166255 1.029584 2.311028 5.447691 1.940691 fitted.predictor.042 1.217094 NA 2.582946 1.165233 1.058728 2.350080 5.477622 1.982258 fitted.predictor.043 1.391996 NA 2.620294 1.164145 1.088655 2.389805 5.507934 2.024570 fitted.predictor.044 1.566899 NA 2.658306 1.162991 1.119382 2.430214 5.538646 2.067639 fitted.predictor.045 1.741802 NA 2.696996 1.161771 1.150928 2.471320 5.569776 2.111475 fitted.predictor.046 1.916705 NA 2.736379 1.160484 1.183221 2.513135 5.601344 2.156089
newdata <- reshape:::rename(newdata, c("0.025quant"="lower", "0.975quant"="upper")) library(grid) ggplot(newdata, aes(y=mean, x=x)) + geom_blank()+ geom_point(data=data.nb, aes(y=y, x=x)) + geom_ribbon(aes(ymin=lower, ymax=upper), fill='blue', alpha=0.2) + geom_line() + theme_classic()+ theme(legend.key.width=unit(2,'lines'), legend.position=c(0,1), legend.justification=c(0,1))
Zero-inflation Poisson (ZIP) regression (count data)
Lets say we wanted to model the abundance of an item ($y$) against a continuous predictor ($x$). As this section is mainly about the generation of artificial data (and not specifically about what to do with the data), understanding the actual details are optional and can be safely skipped. Consequently, I have folded (toggled) this section away.
- the sample size = 20
- the continuous $x$ variable is a random uniform spread of measurements between 1 and 20
- the rate of change in log $y$ per unit of $x$ (slope) = 0.1.
- the value of $x$ when log$y$ equals 0 (when $y$=1)
- to generate the values of $y$ expected at each $x$ value, we evaluate the linear predictor (created by calculating the outer product of the model matrix and the regression parameters). These expected values are then transformed into a scale mapped by (0,$\infty$) by using the log function $e^{linear~predictor}$
- finally, we generate $y$ values by using the expected $y$ values ($\lambda$) as probabilities when drawing random numbers from a Poisson distribution. This step adds random noise to the expected $y$ values and returns only 0's and positive integers.
set.seed(37) #34.5 #4 #10 #16 #17 #26 #The number of samples n.x <- 20 #Create x values that at uniformly distributed throughout the rate of 1 to 20 x <- sort(runif(n = n.x, min = 1, max =20)) mm <- model.matrix(~x) intercept <- 0.6 slope=0.1 #The linear predictor linpred <- mm %*% c(intercept,slope) #Predicted y values lambda <- exp(linpred) #Add some noise and make binomial library(gamlss.dist) #fixed latent binomial y<- rZIP(n.x,lambda, 0.4) #latent binomial influenced by the linear predictor #y<- rZIP(n.x,lambda, 1-exp(linpred)/(1+exp(linpred))) dat.zip <- data.frame(y,x) data.zip <- dat.zip summary(glm(y~x, dat.zip, family="poisson"))
Call:
glm(formula = y ~ x, family = "poisson", data = dat.zip)
Deviance Residuals:
Min 1Q Median 3Q Max
-2.5415 -2.3769 -0.9753 1.1736 3.6380
Coefficients:
Estimate Std. Error z value Pr(>|z|)
(Intercept) 0.67048 0.33018 2.031 0.0423 *
x 0.02961 0.02663 1.112 0.2662
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
(Dispersion parameter for poisson family taken to be 1)
Null deviance: 85.469 on 19 degrees of freedom
Residual deviance: 84.209 on 18 degrees of freedom
AIC: 124.01
Number of Fisher Scoring iterations: 6
plot(glm(y~x, dat.zip, family="poisson"))
library(pscl) summary(zeroinfl(y ~ x | 1, dist = "poisson", data = dat.zip))
Call:
zeroinfl(formula = y ~ x | 1, data = dat.zip, dist = "poisson")
Pearson residuals:
Min 1Q Median 3Q Max
-1.0015 -0.9556 -0.3932 0.9663 1.6195
Count model coefficients (poisson with log link):
Estimate Std. Error z value Pr(>|z|)
(Intercept) 0.70474 0.31960 2.205 0.027449 *
x 0.08734 0.02532 3.449 0.000563 ***
Zero-inflation model coefficients (binomial with logit link):
Estimate Std. Error z value Pr(>|z|)
(Intercept) -0.2292 0.4563 -0.502 0.615
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Number of iterations in BFGS optimization: 13
Log-likelihood: -36.17 on 3 Df
plot(resid(zeroinfl(y ~ x | 1, dist = "poisson", data = dat.zip))~fitted(zeroinfl(y ~ x | 1, dist = "poisson", data = dat.zip)))
library(gamlss) summary(gamlss(y~x,data=dat.zip, family=ZIP))
GAMLSS-RS iteration 1: Global Deviance = 72.4363
GAMLSS-RS iteration 2: Global Deviance = 72.3428
GAMLSS-RS iteration 3: Global Deviance = 72.3428
*******************************************************************
Family: c("ZIP", "Poisson Zero Inflated")
Call: gamlss(formula = y ~ x, family = ZIP, data = dat.zip)
Fitting method: RS()
-------------------------------------------------------------------
Mu link function: log
Mu Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 0.70582 0.31958 2.209 0.04123 *
x 0.08719 0.02533 3.442 0.00311 **
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
-------------------------------------------------------------------
Sigma link function: logit
Sigma Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) -0.2292 0.4563 -0.502 0.622
-------------------------------------------------------------------
No. of observations in the fit: 20
Degrees of Freedom for the fit: 3
Residual Deg. of Freedom: 17
at cycle: 3
Global Deviance: 72.34282
AIC: 78.34282
SBC: 81.33002
*******************************************************************
predict(gamlss(y~x,data=dat.zip, family=ZIP), se.fit=TRUE, what="mu")
GAMLSS-RS iteration 1: Global Deviance = 72.4363 GAMLSS-RS iteration 2: Global Deviance = 72.3428 GAMLSS-RS iteration 3: Global Deviance = 72.3428
$fit
1 2 3 4 5 6 7 8 9 10 11
0.7966905 0.9236189 1.0263933 1.0369823 1.1305358 1.3763304 1.4054417 1.4299603 1.4951229 1.6161339 1.7035853
12 13 14 15 16 17 18 19 20
1.7629994 1.8678543 1.9838052 1.9951833 2.1187071 2.1249555 2.1431616 2.1837285 2.3064727
$se.fit
1 2 3 4 5 6 7 8 9 10 11
0.3517894 0.3089432 0.2758980 0.2726011 0.2445792 0.1856062 0.1807322 0.1770893 0.1696661 0.1656458 0.1710016
12 13 14 15 16 17 18 19 20
0.1783138 0.1973009 0.2251987 0.2282363 0.2637860 0.2656903 0.2712879 0.2840032 0.3241532
$$p(y \mid \theta,\lambda) = \left\{ \begin{array}{l l} \theta + (1-\theta)\times \text{Pois}(0|\lambda) & \quad \text{if $y_i=0$ and}\\ (1-\theta)\times \text{Pois}(y_i|\lambda) & \quad \text{if $y_i>0$} \end{array} \right.$ $$ $$ \begin{align} log(\lambda_{ij})&=\beta_0+\beta_1x_1 + \sum^j_{J=1} \gamma U_j\\ \end{align} $$
library(pscl) summary(zeroinfl(y ~ x | 1, dist = "poisson", data = dat.zip))
Call:
zeroinfl(formula = y ~ x | 1, data = dat.zip, dist = "poisson")
Pearson residuals:
Min 1Q Median 3Q Max
-1.0015 -0.9556 -0.3932 0.9663 1.6195
Count model coefficients (poisson with log link):
Estimate Std. Error z value Pr(>|z|)
(Intercept) 0.70474 0.31960 2.205 0.027449 *
x 0.08734 0.02532 3.449 0.000563 ***
Zero-inflation model coefficients (binomial with logit link):
Estimate Std. Error z value Pr(>|z|)
(Intercept) -0.2292 0.4563 -0.502 0.615
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Number of iterations in BFGS optimization: 13
Log-likelihood: -36.17 on 3 Df
summary(zeroinfl(y ~ x | x, dist = "poisson", data = dat.zip))
Call:
zeroinfl(formula = y ~ x | x, data = dat.zip, dist = "poisson")
Pearson residuals:
Min 1Q Median 3Q Max
-1.2761 -0.7814 -0.5933 0.9078 2.1317
Count model coefficients (poisson with log link):
Estimate Std. Error z value Pr(>|z|)
(Intercept) 0.61113 0.33001 1.852 0.064044 .
x 0.09389 0.02574 3.647 0.000265 ***
Zero-inflation model coefficients (binomial with logit link):
Estimate Std. Error z value Pr(>|z|)
(Intercept) -2.7305 1.8897 -1.445 0.148
x 0.2170 0.1461 1.485 0.137
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Number of iterations in BFGS optimization: 17
Log-likelihood: -34.41 on 4 Df
library(gamlss) summary(gamlss(y~x,data=dat.zip, family=ZIP))
GAMLSS-RS iteration 1: Global Deviance = 72.4363
GAMLSS-RS iteration 2: Global Deviance = 72.3428
GAMLSS-RS iteration 3: Global Deviance = 72.3428
*******************************************************************
Family: c("ZIP", "Poisson Zero Inflated")
Call: gamlss(formula = y ~ x, family = ZIP, data = dat.zip)
Fitting method: RS()
-------------------------------------------------------------------
Mu link function: log
Mu Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 0.70582 0.31958 2.209 0.04123 *
x 0.08719 0.02533 3.442 0.00311 **
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
-------------------------------------------------------------------
Sigma link function: logit
Sigma Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) -0.2292 0.4563 -0.502 0.622
-------------------------------------------------------------------
No. of observations in the fit: 20
Degrees of Freedom for the fit: 3
Residual Deg. of Freedom: 17
at cycle: 3
Global Deviance: 72.34282
AIC: 78.34282
SBC: 81.33002
*******************************************************************
dat.zip.list <- with(dat.zip,list(Y=y, X=x,N=nrow(data.zip), z=ifelse(y==0,0,1))) modelString=" model { for (i in 1:N) { z[i] ~ dbern(one.minus.theta) Y[i] ~ dpois(lambda[i]) lambda[i] <- z[i]*eta[i] log(eta[i]) <- beta0 + beta1*X[i] } one.minus.theta <- 1-theta logit(theta) <- gamma0 beta0 ~ dnorm(0,1.0E-06) beta1 ~ dnorm(0,1.0E-06) gamma0 ~ dnorm(0,1.0E-06) } " library(R2jags) dat.zip.jags <- jags(model=textConnection(modelString), data=dat.zip.list, inits=NULL, param=c('beta0','beta1', 'gamma0','theta'), n.chain=3, n.iter=20000, n.thin=10, n.burnin=10000)
Compiling model graph Resolving undeclared variables Allocating nodes Graph Size: 149 Initializing model
print(dat.zip.jags)
Inference for Bugs model at "5", fit using jags,
3 chains, each with 20000 iterations (first 10000 discarded), n.thin = 10
n.sims = 3000 iterations saved
mu.vect sd.vect 2.5% 25% 50% 75% 97.5% Rhat n.eff
beta0 0.711 0.308 0.101 0.504 0.719 0.915 1.318 1.001 3000
beta1 0.086 0.025 0.037 0.070 0.085 0.102 0.133 1.001 3000
gamma0 -0.206 0.461 -1.129 -0.502 -0.194 0.113 0.683 1.001 3000
theta 0.451 0.109 0.244 0.377 0.452 0.528 0.664 1.001 3000
deviance 75.580 2.443 72.827 73.793 74.952 76.659 81.836 1.003 1300
For each parameter, n.eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor (at convergence, Rhat=1).
DIC info (using the rule, pD = var(deviance)/2)
pD = 3.0 and DIC = 78.6
DIC is an estimate of expected predictive error (lower deviance is better).
library(rstan) modelString=" functions { real zero_inflated_poisson_log(int y, real eta, real eta_zi) { if (y == 0) { return log_sum_exp(bernoulli_logit_log(1, eta_zi), bernoulli_logit_log(0, eta_zi) + poisson_log_log(0, eta)); } else { return bernoulli_logit_log(0, eta_zi) + poisson_log_log(y, eta); } } } data { int<lower=1> n; int<lower=1> nX; int y[n]; matrix [n*2,nX] X; } parameters { vector [nX] beta; real<lower=0> shape; } transformed parameters { vector [n*2] eta; eta<-X*beta; } model { beta ~ normal(0,1000); for (i in 1:n) { y[i] ~ zero_inflated_poisson(eta[i], eta[i+n]); } } " Xmat <- model.matrix(~x,data=data.zip) dat.list <- with(data.zip, list(y = y, X = rbind(Xmat,Xmat), nX=ncol(Xmat), n = nrow(data.zip))) library(rstan) dat.rstan <- stan(data=dat.list, model_code=modelString, chains=3, iter=1000, warmup=500, thin=2, save_dso=TRUE )
SAMPLING FOR MODEL '983801421387f281f0182fb32509705d' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.454001 seconds (Warm-up) # 0.115789 seconds (Sampling) # 0.56979 seconds (Total) # SAMPLING FOR MODEL '983801421387f281f0182fb32509705d' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.155689 seconds (Warm-up) # 0.035454 seconds (Sampling) # 0.191143 seconds (Total) # SAMPLING FOR MODEL '983801421387f281f0182fb32509705d' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.200173 seconds (Warm-up) # 0.322706 seconds (Sampling) # 0.522879 seconds (Total) #
print(dat.rstan, pars=c('beta'))
Inference for Stan model: 983801421387f281f0182fb32509705d.
3 chains, each with iter=1000; warmup=500; thin=2;
post-warmup draws per chain=250, total post-warmup draws=750.
mean se_mean sd 2.5% 25% 50% 75% 97.5% n_eff Rhat
beta[1] 0.41 0.14 0.24 0.03 0.22 0.4 0.55 0.86 3 1.50
beta[2] 0.10 0.01 0.02 0.06 0.09 0.1 0.11 0.13 4 1.27
Samples were drawn using NUTS(diag_e) at Thu Feb 25 13:24:10 2016.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).
## dat.rstan.model <- stan_model(model_code=modelString) ## dat.rstan <- sampling(dat.rstan.model,data=dat.list,chains=3,iter=1000,warmup=500,thin=2)
library(brms) dat.brm <- brm(y~x, data=data.zip, family='zero_inflated_poisson', prior=c(set_prior('normal(0,1000)', class='b')), chains=3, iter=1000, warmup=500, thin=2 )
SAMPLING FOR MODEL 'zero_inflated_poisson(log) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.048984 seconds (Warm-up) # 0.03777 seconds (Sampling) # 0.086754 seconds (Total) # SAMPLING FOR MODEL 'zero_inflated_poisson(log) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.04523 seconds (Warm-up) # 0.041655 seconds (Sampling) # 0.086885 seconds (Total) # SAMPLING FOR MODEL 'zero_inflated_poisson(log) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.042881 seconds (Warm-up) # 0.042045 seconds (Sampling) # 0.084926 seconds (Total) #
summary(dat.brm)
Family: zero_inflated_poisson (log)
Formula: y ~ x
Data: data.zip (Number of observations: 20)
Samples: 3 chains, each with iter = 1000; warmup = 500; thin = 2;
total post-warmup samples = 750
WAIC: Not computed
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
Intercept 0.4 0.36 -0.38 1.08 495 1
x 0.1 0.03 0.05 0.16 460 1
Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
is a crude measure of effective sample size, and Rhat is the potential
scale reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
/* zero-inflated poisson log-PDF of a single response
* Args:
* y: the response value
* eta: linear predictor for poisson part
* eta_zi: linear predictor for zero-inflation part
* Returns:
* a scalar to be added to the log posterior
*/
real zero_inflated_poisson_log(int y, real eta, real eta_zi) {
if (y == 0) {
return log_sum_exp(bernoulli_logit_log(1, eta_zi),
bernoulli_logit_log(0, eta_zi) +
poisson_log_log(0, eta));
} else {
return bernoulli_logit_log(0, eta_zi) +
poisson_log_log(y, eta);
}
}
}
data {
int<lower=1> N; // number of observations
int<lower=1> N_trait; // number of obs / 2
int Y[N_trait]; // response variable
int<lower=1> K; // number of fixed effects
matrix[N, K] X; // FE design matrix
vector[K] X_means; // column means of X
}
transformed data {
}
parameters {
real temp_Intercept; // temporary Intercept
vector[K] b; // fixed effects
}
transformed parameters {
vector[N] eta; // linear predictor
// compute linear predictor
eta <- X * b + temp_Intercept;
}
model {
// prior specifications
b ~ normal(0,1000);
// likelihood contribution
for (n in 1:N_trait) {
Y[n] ~ zero_inflated_poisson(eta[n], eta[n + N_trait]);
}
}
generated quantities {
real b_Intercept; // fixed effects intercept
b_Intercept <- temp_Intercept - dot_product(X_means, b);
}
standata(dat.brm)
$N
[1] 40
$Y
[1] 0 3 1 4 5 1 5 6 4 0 0 0 0 4 0 0 9 0 0 12
$N_trait
[1] 20
$K
[1] 1
$X
x
1 -9.45822567
2 -8.00251059
3 -6.82381320
4 -6.70236995
5 -5.62942498
6 -2.81045852
7 -2.47658683
8 -2.19538785
9 -1.44805234
10 -0.06020261
11 0.94275959
12 1.62416640
13 2.82672610
14 4.15654241
15 4.28703487
16 5.70370304
17 5.77536504
18 5.98416729
19 6.44941992
20 7.85714787
21 -9.45822567
22 -8.00251059
23 -6.82381320
24 -6.70236995
25 -5.62942498
26 -2.81045852
27 -2.47658683
28 -2.19538785
29 -1.44805234
30 -0.06020261
31 0.94275959
32 1.62416640
33 2.82672610
34 4.15654241
35 4.28703487
36 5.70370304
37 5.77536504
38 5.98416729
39 6.44941992
40 7.85714787
$X_means
x
10.50042
# include zero-inflated intercept dat.brm <- brm(y~0+main + spec + main:x, data=data.zip, family='zero_inflated_poisson', prior=c(set_prior('normal(0,1000)', class='b')), chains=3, iter=1000, warmup=500, thin=2 )
SAMPLING FOR MODEL 'zero_inflated_poisson(log) brms-model' NOW (CHAIN 1). Chain 1, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 1, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 1, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 1, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 1, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 1, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 1, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 1, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 1, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 1, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 1, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 1, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.163529 seconds (Warm-up) # 0.065766 seconds (Sampling) # 0.229295 seconds (Total) # SAMPLING FOR MODEL 'zero_inflated_poisson(log) brms-model' NOW (CHAIN 2). Chain 2, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 2, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 2, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 2, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 2, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 2, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 2, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 2, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 2, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 2, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 2, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 2, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.117409 seconds (Warm-up) # 0.059019 seconds (Sampling) # 0.176428 seconds (Total) # SAMPLING FOR MODEL 'zero_inflated_poisson(log) brms-model' NOW (CHAIN 3). Chain 3, Iteration: 1 / 1000 [ 0%] (Warmup) Chain 3, Iteration: 100 / 1000 [ 10%] (Warmup) Chain 3, Iteration: 200 / 1000 [ 20%] (Warmup) Chain 3, Iteration: 300 / 1000 [ 30%] (Warmup) Chain 3, Iteration: 400 / 1000 [ 40%] (Warmup) Chain 3, Iteration: 500 / 1000 [ 50%] (Warmup) Chain 3, Iteration: 501 / 1000 [ 50%] (Sampling) Chain 3, Iteration: 600 / 1000 [ 60%] (Sampling) Chain 3, Iteration: 700 / 1000 [ 70%] (Sampling) Chain 3, Iteration: 800 / 1000 [ 80%] (Sampling) Chain 3, Iteration: 900 / 1000 [ 90%] (Sampling) Chain 3, Iteration: 1000 / 1000 [100%] (Sampling)# # Elapsed Time: 0.132163 seconds (Warm-up) # 0.064132 seconds (Sampling) # 0.196295 seconds (Total) #
summary(dat.brm)
Family: zero_inflated_poisson (log)
Formula: y ~ 0 + main + spec + main:x
Data: data.zip (Number of observations: 20)
Samples: 3 chains, each with iter = 1000; warmup = 500; thin = 2;
total post-warmup samples = 750
WAIC: Not computed
Fixed Effects:
Estimate Est.Error l-95% CI u-95% CI Eff.Sample Rhat
main 0.66 0.32 0.00 1.24 384 1.01
spec -0.26 0.47 -1.18 0.68 601 1.00
main:x 0.09 0.03 0.04 0.14 413 1.00
Samples were drawn using sampling(NUTS). For each parameter, Eff.Sample
is a crude measure of effective sample size, and Rhat is the potential
scale reduction factor on split chains (at convergence, Rhat = 1).
stancode(dat.brm)
functions {
/* zero-inflated poisson log-PDF of a single response
* Args:
* y: the response value
* eta: linear predictor for poisson part
* eta_zi: linear predictor for zero-inflation part
* Returns:
* a scalar to be added to the log posterior
*/
real zero_inflated_poisson_log(int y, real eta, real eta_zi) {
if (y == 0) {
return log_sum_exp(bernoulli_logit_log(1, eta_zi),
bernoulli_logit_log(0, eta_zi) +
poisson_log_log(0, eta));
} else {
return bernoulli_logit_log(0, eta_zi) +
poisson_log_log(y, eta);
}
}
}
data {
int<lower=1> N; // number of observations
int<lower=1> N_trait; // number of obs / 2
int Y[N_trait]; // response variable
int<lower=1> K; // number of fixed effects
matrix[N, K] X; // FE design matrix
vector[K] X_means; // column means of X
}
transformed data {
}
parameters {
vector[K] b; // fixed effects
}
transformed parameters {
vector[N] eta; // linear predictor
// compute linear predictor
eta <- X * b;
}
model {
// prior specifications
b ~ normal(0,1000);
// likelihood contribution
for (n in 1:N_trait) {
Y[n] ~ zero_inflated_poisson(eta[n], eta[n + N_trait]);
}
}
generated quantities {
}
standata(dat.brm)
$N
[1] 40
$Y
[1] 0 3 1 4 5 1 5 6 4 0 0 0 0 4 0 0 9 0 0 12
$N_trait
[1] 20
$K
[1] 3
$X
main spec main:x
1 1 0 1.042191
2 1 0 2.497906
3 1 0 3.676603
4 1 0 3.798047
5 1 0 4.870992
6 1 0 7.689958
7 1 0 8.023830
8 1 0 8.305029
9 1 0 9.052364
10 1 0 10.440214
11 1 0 11.443176
12 1 0 12.124583
13 1 0 13.327143
14 1 0 14.656959
15 1 0 14.787451
16 1 0 16.204120
17 1 0 16.275782
18 1 0 16.484584
19 1 0 16.949836
20 1 0 18.357564
21 0 1 0.000000
22 0 1 0.000000
23 0 1 0.000000
24 0 1 0.000000
25 0 1 0.000000
26 0 1 0.000000
27 0 1 0.000000
28 0 1 0.000000
29 0 1 0.000000
30 0 1 0.000000
31 0 1 0.000000
32 0 1 0.000000
33 0 1 0.000000
34 0 1 0.000000
35 0 1 0.000000
36 0 1 0.000000
37 0 1 0.000000
38 0 1 0.000000
39 0 1 0.000000
40 0 1 0.000000
$X_means
main spec main:x
0.500000 0.500000 5.250208
